Leukocytapheresis Changes Functional Characteristics of Circulating Neutrophils in Humans: Mechanistic Implications
Koji Tanaka, Toshimitsu Araki, Shigeyuki Yoshiyama, Yoshiki Okita, Satoru Kondo, Yuji Toiyama, Mikihiro Inoue, Masaki Ohi, Minako Kobayashi, Yasuhiro Inoue, Keiichi Uchida, Yasuhiko Mohri, Chikao Miki and Masato Kusunoki
DOI10.4172/2472-1891.100017
Koji Tanaka1*, Toshimitsu Araki1, Shigeyuki Yoshiyama2, Yoshiki Okita1, Satoru Kondo1, Yuji Toiyama1, Mikihiro Inoue1, Masaki Ohi1, Minako Kobayashi1, Yasuhiro Inoue1, Keiichi Uchida1, Yasuhiko Mohri1, Chikao Miki3 and Masato Kusunoki1
1Department of Gastrointestinal and Pediatric Surgery, Mie University Graduate School of Medicine, Mie, Japan
2Department of Surgery, Kuwana West Medical Center, Mie, Japan
3Department of Surgery, IGA City General Hospital, Mie, Japan
- *Corresponding Author:
- Koji Tanaka
Department of Gastrointestinal and Pediatric Surgery, Mie University
Graduate School of Medicine, 2-174 Edobashi, Tsu,Mie 514-8507, Japan
Tel: +81-59-231-5294
Fax: +81-59-232-6968
E-mail: qouji@clin.medic.mie-u.ac.jp
Received date: October 13, 2015;Accepted date: January 28 2016; Published date: February 05,2016
Citation: Tanaka K, Araki T, Yoshiyama S, et al. Leukocytapheresis Changes Functional Characteristics of Circulating Neutrophils in Humans: Mechanistic Implications. Int J Dig Dis. 2016, 2:1 doi:10.4172/2472-1891.100017.
Abstract
Background and aim: Postoperative leukocytapheresis (LCAP) reduces surgical site infection (SSI) in ulcerative colitis (UC) patients undergoing proctocolectomy with ileal pouch–anal anastomosis (IPAA). To clarify the effect of postoperative LCAP on circulating neutrophils, we investigated neutrophil function before and after LCAP in UC patients undergoing proctocolectomy with IPAA.
Method: Circulating neutrophils were obtained from 29 UC patients undergoing IPAA before and after postoperative LCAP. The phagocytic activity was measured using Escherichia coli BioParticles. Neutrophil viability after co-culture with E. coli was measured by flow cytometry. Inflammatory cytokines in patient serum and supernatants after co-culture of neutrophils with E. coli were measured by enzyme-linked immunosorbent assay.
Results: No difference in phagocytic activity was found between preand post-LCAP neutrophils. The percentage of necrotic neutrophils was significantly decreased in post-LCAP neutrophils cocultured with E. coli. Serum neutrophil elastase (NE) after LCAP was significantly increased. No difference in cytokine production was found between pre- and post-LCAP neutrophils cocultured with E. coli. NE and tumor necrosis factor (TNF)-α levels in supernatants of post-LCAP neutrophils cocultured with E. coli were higher in patients with longer operating time and higher postoperative neutrophil count, respectively. Phagocytic activity of post-LCAP neutrophils was lower in the longer operating time group. Serum TNF-α level after LCAP was higher in the SSI group.
Conclusion: Postoperative LCAP decreases the subset of circulating neutrophils, which induces necrosis against bacterial infection. This suggests a possible mechanism of prevention of SSI.
Introduction
Neutrophils are the most abundant population of circulating white blood cells and the host’s first line of defense against invading pathogens [1]. Under normal conditions, circulating neutrophils have a 6–12 h half-life and are functionally quiescent. During inflammation, neutrophils arrive rapidly at the sites of infection and injury, enhance antimicrobial activity, and die within the infiltrated tissue [2]. They play important roles in the innate immune system, and significant neutrophil responses are observed in systemic inflammation such as trauma, burn injury, sepsis, and major surgery [3].
However, the dysregulation of neutrophil responses such as excessive or uncontrolled release of proinflammatory cytokines can cause host tissue or organ damage during severe sepsis, and autoimmune diseases [4, 5].
Leukocyte removal therapy (LRT), leukocytapheresis (LCAP), or granulocyte/monocyte apheresis (GMA) is a method of therapeutic apheresis that removes peripheral leukocytes by via extracorporeal circulation. It has been used as nonpharmacological treatment in ulcerative colitis (UC) and Crohn’s disease (CD), and has shown excellent short-term efficacy [6, 7].
LCAP using Cellsorba, a column of polyethylene telephthalate fibers (Asahi-Kasei Medical, Tokyo, Japan), has been reported to remove ~100% of granulocytes/monocytes, ~60% of lymphocytes, and ~50% of platelets from both the inlet and outlet of the Cellsorba apparatus [8, 9]. Although the exact mechanisms of LRT have not been fully explored, their potential mechanisms have been reported, which include a reduction in the number of activated leukocytes or proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8 [10-13].
Surgical site infection (SSI) remains one of the most common causes of morbidity in major surgery, which prolongs hospitalization and increases medical costs [14]. The Japanese prospective multicenter surveillance showed that 19.5% of patients with UC developed SSI after surgery [15].
In a case-control study, we retrospectively compared the incidence of SSIs between patients undergoing proctocolectomy with ileal pouch–anal anastomosis (IPAA) (n=29) and those undergoing IPAA with postoperative LCAP (n=43). Postoperative LCAP significantly reduced SSIs in UC patients undergoing proctocolectomy with IPAA (p<0.01) [16]. In a multicenter prospective study which we conducted, the incidence of SSIs was compared between UC patients undergoing surgery (control group; n=103) and those undergoing surgery with postoperative LCAP (n=40). SSIs occurred in 4 of 37 patients (10.8%) in the LCAP group, whereas they occurred in 29 of 103 patients (28.2%) in the control group, although the difference didn’t reach statistical significance (p=0.069) [17].
These clinical observations suggest that LCAP removes proinflammatory cytokine-producing activated leukocytes in postoperative systemic inflammation due to major colorectal surgery, and might suppress the occurrence of SSI. We have reported an association between neutrophil dysfunction and postoperative infectious complications [18-21].
Neutrophils are the most abundant cells among circulating leukocytes and can be the most frequently removed by LCAP [8, 9]. Thus, we focused on the functions of circulating neutrophils isolated before and after LCAP, to clarify the therapeutic mechanisms of postoperative LCAP on the occurrence of SSIs in UC patients after surgery.
Materials and Method
Patients
Forty-three patients with UC undergoing IPAA received postoperative LCAP at the Department of Gastrointestinal and Pediatric Surgery of Mie University Graduate School of Medicine from 2004 to 2008. Diagnosis of UC was based on the clinical, radiographic, endoscopic, surgical, and histopathological data. Before surgery, patients received various medical treatments including anti-inflammatory drugs (aminosalicylates, corticosteroids) or immunosuppressant drugs (azathioprine, mercaptopurine, cyclosporine), or both. Postoperative LCAP was performed once within 2 hours after surgery. Patients with clinical symptoms of sepsis were excluded from the study. Patients with unstable hemodynamics following surgery were also excluded according to the protocol. Written informed consent was obtained from all patients before the start of this study, which were performed in accordance with the Helsinki Declaration and were approved by the Institutional Review Board (No. 474).
Neutrophil isolation
Pre- and post-LCAP neutrophils were isolated from the same patients before and after surgery. Neutrophil isolation was based on a previously described procedure [20]. The blood samples (10 ml) were diluted with an equal volume of phosphate-buffered saline (PBS), layered over a volume of Ficoll-Paque PLUS (Stemcell Technologies Inc., Vancouver, BC, Canada) and centrifuged at 600 × g for 30 min at room temperature. After removal of mononuclear cells, plasma, and Ficoll-Paque PLUS, the pellet was resuspended in 12 mL ammonium chloride solution to lyse the red blood cells, washed twice in PBS, and resuspended in PBS. Neutrophils were washed twice with PBS and resuspended in TCM-10 [RPMI 1640 medium supplemented with 10% fetal bovine serum, 5 × 10–5 M 2-mercaptoethanol, and 10 mM 4-(2-hydroxyethyl)- 1-piperazine ethanesulfonic acid]. Cell preparations were tested for viability (>99%) by trypan blue exclusion and for purity (>95% CD13+, CD33+ granulocytes) by flow cytometry.
Phagocytic activity
Ex vivo phagocytic activity of circulating neutrophils against Escherichia coli was compared between pre- and post-LCAP neutrophils isolated from identical patients.
Neutrophil phagocytosis was measured using fluorescent bioparticles [Alexa Fluor 488-conjugated E. coli and opsonizing reagents according to the manufacturer’s instructions (Molecular Probes, Eugene, OR, USA)]. To opsonize the bacterial bioparticles, the reconstituted opsonizing reagent and bacterial bioparticles (5 × 105) were mixed, vortexed, and incubated for 1 h at 37°C in a humidified 5% CO2 incubator. After two washes with PBS, separated by low-speed centrifugation (1500 × g, 15 min, 4°C), the pellets were incubated with neutrophils (5 × 105) in 1 mL medium without antibiotics for 3 h at 37°C in a humidified 5% CO2 incubator. After 3 h incubation, phagocytic activity of neutrophils against E. coli was analyzed using a FACScan (Becton–Dickinson, San Jose, CA, USA) and 10,000 events were counted per sample.
Preparation of E. coli
Escherichia coli (ATCC25922) was purchased from American Type Culture Collection (Manassas, VA, USA), and cultured in Dulbecco’s Modified Eagle’s Medium (Invitrogen, Tokyo, Japan) for 10 h at 37°C. The culture medium was centrifuged at 1,700 × g for 10 min at room temperature to obtain a pellet, which was washed twice and resuspended in sterile normal saline. An aliquot (100 μL) of the suspension was serially diluted with sterile saline, plated on Agar-B (Qbiogene, Carlsbad, CA, USA), and incubated for 18 h to determine the bacterial concentration. The remainder was stored at −80°C until use.
Co-culture of neutrophils with E. coli
Just before use, the E. coli suspension was diluted in medium to achieve a final concentration of 2.5 × 106 cfu/mL. After opsonization of E. coli using autologous serum for 30 min at 37°C, neutrophils (2.5 × 105 /mL) were cocultured in 1 mL medium without antibiotics for 3 h at 37°C in a humidified 5% CO2 incubator. After centrifugation of the cells at 600 × g for 10 min at 4°C, the supernatants were collected to analyze cytokine production, neutrophil elastase (NE), and neutrophil viability.
Neutrophil viability
Ex vivo viability of circulating neutrophils against E. coli was compared between pre- and post-LCAP neutrophils isolated from identical patients. To evaluate neutrophil viability after co-culture with E. coli, an apoptosis assay was performed [20, 21].
Apoptosis of neutrophils after co-culture with E. coli was quantified by flow cytometry using an Annexin V Apoptosis Detection Kit (BioVision Research Products, Palo Alto, CA, USA). Neutrophils were incubated in 1X binding buffer to which 5 μL Annexin V–fluorescein isothiocyanate (FITC) and 5 μL propidium iodide (PI) were added. After incubation at room temperature for 5 min in the dark, the cells were analyzed using a FACScan and 10,000 events were counted per sample.
The combination of Annexin V–FITC and PI was able to distinguish between early apoptotic cells (Annexin V+, PI–), necrotic cells (Annexin V–, PI+), late apoptotic or secondary necrotic cells (Annexin V+, PI+), and viable cells (Annexin V–, PI–).
Enzyme-linked immunosorbent assay (ELISA)
Cytokines in patients’ serum before and after LCAP and supernatants after co-culture of pre- and post-LCAP neutrophils with E. coli were measured by ELISA according to the manufacturer’s instructions. Cytokines included TNF-α, IL-1 receptor antagonist (IL-1Ra), IL-6, IL-8, and NE. TNF-α, IL-1Ra, IL-6, and IL-8 ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA). NE ELISA kit was purchased from Immundiagnostik (Bensheim, Germany). The detection limits of TNF-α, IL-1Ra, IL-6, IL-8, and NE were 1, 2, 0.01, and 2 pg/mL and 0.12 ng/mL, respectively, and levels below these limits were considered undetectable. The level of each cytokine was evaluated in duplicate and the average cytokine value was computed.
Statistical analysis
All statistical analyses were done using JMP version 5 (SAS Institute, Cary, NC, USA). Associations between continuous and categorical variables were evaluated using the Mann–Whitney U test or paired t test when appropriate. P<0.05 was considered statistically significant.
Results
Patient characteristics
Twenty-nine UC patients undergoing IPAA received postoperative LCAP. Patient characteristics are shown in Table 1. The mean age at diagnosis was 27 years (range 15–54 years) and the mean age at surgery was 33 years (range 16–61 years). The disease duration was 6.9 years (range 0.1–28 years). Left-sided colitis was in 6/29 (21%) patients and pan-colitis in 23/29 (79%) patients.The disease severity of colitis was classified as mild (7; 24%), moderate (11; 38%), and severe (4; 38%).
| Patient characteristics | |
|---|---|
| Variables | Patients (n=29) Number |
| Gender | |
| Male | 19 |
| Female | 10 |
| Age at diagnosis | 27( 15-54) |
| Age at surgery | 33(16-61) |
| Disease duration | 6.9 (0.1-28) |
| Disease severity | |
| mild | 7 |
| moderate | 11 |
| severe | 4 |
| Disease extent | |
| Left-sided colitis | 6 |
| Pan- colitis | 23 |
| Malts grading system | |
| 1 | 7 |
| 2 | 11 |
| 3 | 8 |
| 4 | 4 |
| Total steroid dose (mg) | 17285 (800-70000) |
| Use of immunosuppressants | |
| Yes | 5 |
| No | 24 |
| Operation time (min) | 262.4 (118-496) |
| Blood loss (ml) | 307.9 (17-826) |
Table 1: Patient characteristics.
Time course change in WBC, neutrophil, and platelet count
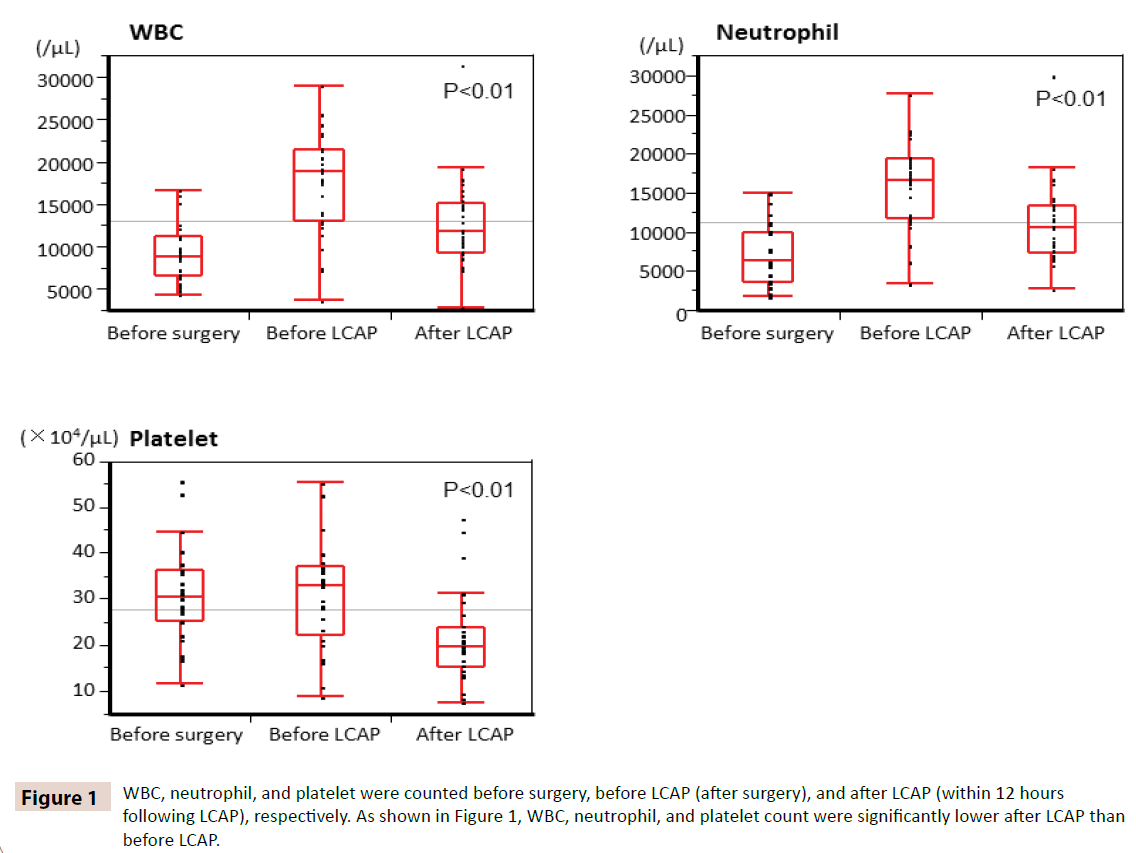
WBC, neutrophil, and platelet were counted before surgery, before LCAP (after surgery), and after LCAP (within 12 hours following LCAP), respectively. As shown in Figure 1, WBC, neutrophil, and platelet count were significantly lower after LCAP than before LCAP. All patients receiving postoperative LCAP had no hematologic adverse effect.
Phagocytic activity of neutrophils against E. coli
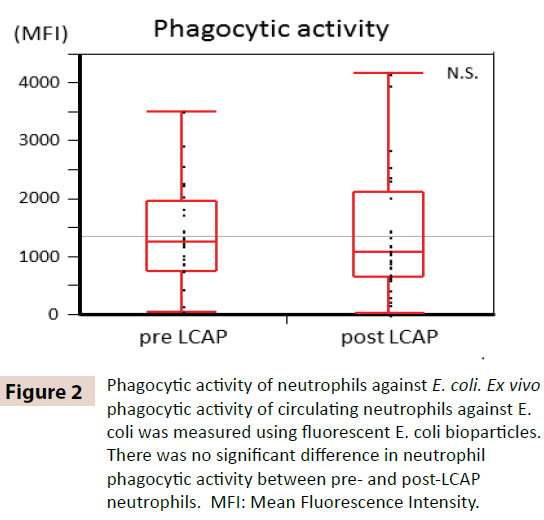
To clarify whether LCAP influences the phagocytic activity of circulating neutrophils, phagocytic activity against E. coli was compared between pre- and post-LCAP neutrophils. As shown in Figure 2, No significant difference in neutrophil phagocytic activity (mean fluorescent intensity) against E. coli was observed between pre-LCAP (median 1259.4, range 50.8–3505.6) and post- LCAP (median 1077.6, range 24.4–4152.1) neutrophils (P=0.72). These results suggest that LCAP does not affect the phagocytic activity of circulating neutrophils against E. coli.
Figure 2: Phagocytic activity of neutrophils against E. coli. Ex vivo phagocytic activity of circulating neutrophils against E. coli was measured using fluorescent E. coli bioparticles. There was no significant difference in neutrophil phagocytic activity between pre- and post-LCAP neutrophils. MFI: Mean Fluorescence Intensity.
Neutrophil viability after co-culture with E. coli
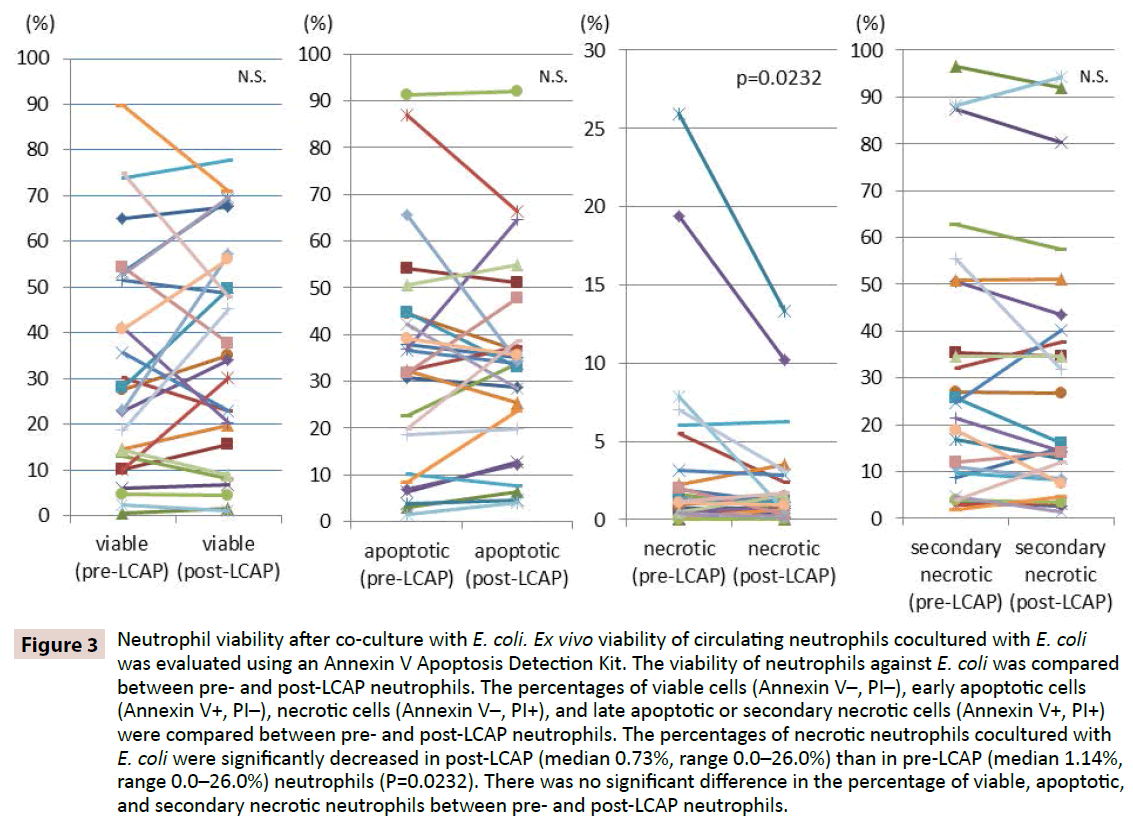
To clarify whether LCAP influences the response of neutrophils against bacterial infection, viability of neutrophils against E. coli was compared between pre- and post-LCAP neutrophils. The percentages of viable cells (Annexin V–, PI–), early apoptotic cells (Annexin V+, PI–), necrotic cells (Annexin V–, PI+), and late apoptotic or secondary necrotic cells (Annexin V+, PI+) were compared between pre- and post-LCAP neutrophils. As shown in Figure 3, the median percentage of viable neutrophils cocultured with E. coli was higher in post-LCAP (median 34.2%, range 1.16– 77.7%) than in pre-LCAP (median 27.9%, range 0.53–89.7%) neutrophils, although the difference did not reach statistical significance(P=0.82). The percentage of necrotic neutrophils cocultured with E. coli was significantly decreased in post-LCAP (median 0.73%, range 0.0–26.0%) than in pre-LCAP (median 1.14%, range 0.0–26.0%) neutrophils (P=0.0232).
Figure 3: Neutrophil viability after co-culture with E. coli. Ex vivo viability of circulating neutrophils cocultured with E. coli was evaluated using an Annexin V Apoptosis Detection Kit. The viability of neutrophils against E. coli was compared between pre- and post-LCAP neutrophils. The percentages of viable cells (Annexin V–, PI–), early apoptotic cells (Annexin V+, PI–), necrotic cells (Annexin V–, PI+), and late apoptotic or secondary necrotic cells (Annexin V+, PI+) were compared between pre- and post-LCAP neutrophils. The percentages of necrotic neutrophils cocultured with E. coli were significantly decreased in post-LCAP (median 0.73%, range 0.0–26.0%) than in pre-LCAP (median 1.14%, range 0.0–26.0%) neutrophils (P=0.0232). There was no significant difference in the percentage of viable, apoptotic, and secondary necrotic neutrophils between pre- and post-LCAP neutrophils.
No significant difference in the percentage of apoptotic neutrophils cocultured with E. coli was observed between pre- LCAP (median 32.3%, range 1.51–91.2%) and post-LCAP (median 33.8%, range 4.0–92.1%) neutrophils (P=0.57). No significant difference in the percentage of late apoptotic or secondary necrotic neutrophils cocultured with E. coli was also observed between pre- LCAP (median 23.1%, range 1.81–96.5%) and post- LCAP (median 16.1%, range 1.44–94.3%) neutrophils (P=0.13).
These results suggest that LCAP may decrease the distinct subset of circulating neutrophils that induce necrosis against bacterial infection, and increase the number of viable (probably unprimed) neutrophils in the systemic circulation.
Cytokine production by neutrophils after coculture with E. coli
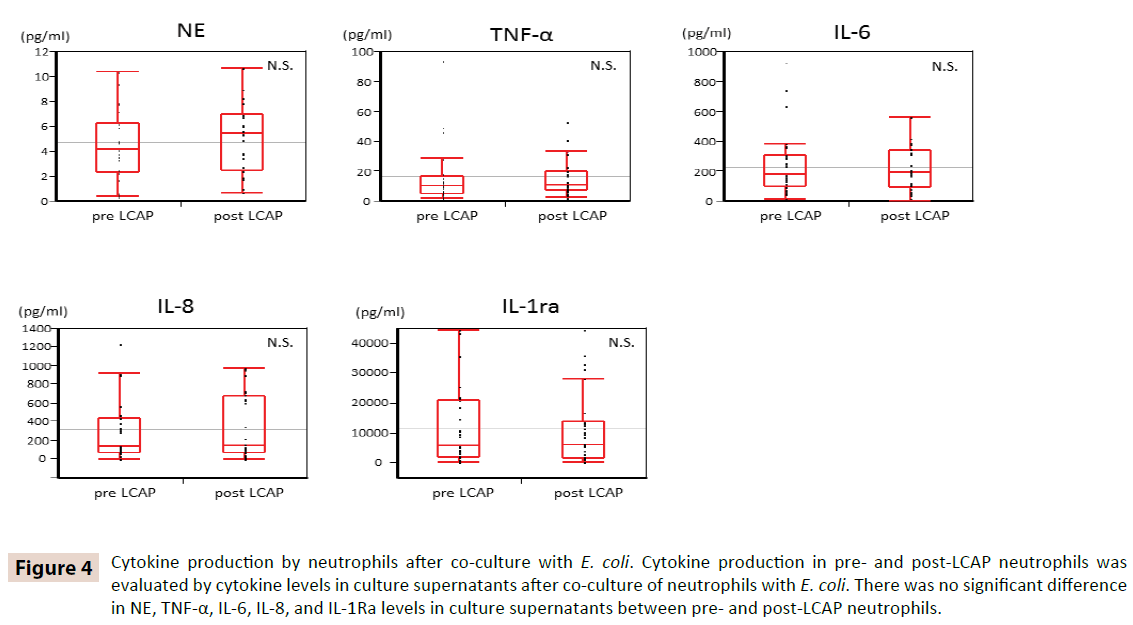
To clarify whether LCAP influences cytokine production by circulating neutrophils, cytokine levels in culture supernatants after co-culture with E. coli were compared between pre- and post- LCAP neutrophils. As shown in Figure 4, there was no significant difference in NE, TNF-α, IL-6, IL-8, and IL-1Ra levels in culture supernatants between pre- and post-LCAP neutrophils. These results suggest that LCAP does not affect cytokine production of circulating neutrophils against E. coli.
Figure 4: Cytokine production by neutrophils after co-culture with E. coli. Cytokine production in pre- and post-LCAP neutrophils was evaluated by cytokine levels in culture supernatants after co-culture of neutrophils with E. coli. There was no significant difference in NE, TNF-α, IL-6, IL-8, and IL-1Ra levels in culture supernatants between pre- and post-LCAP neutrophils.
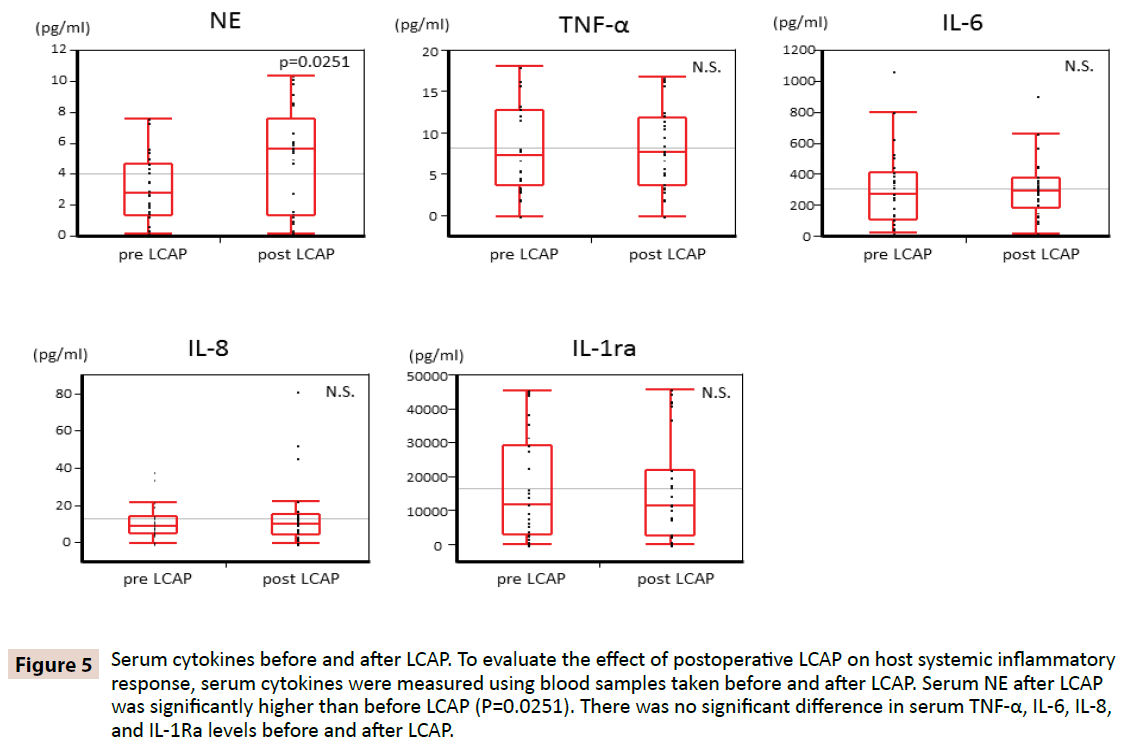
Serum cytokines before and after LCAP
To clarify whether LCAP influences host systemic inflammatory response, serum cytokines were measured using blood samples taken before and after LCAP. As shown in Figure 5, serum NE after LCAP (median 5.62 pg/mL, range 0.16–10.4 pg/mL) was significantly higher than that before LCAP (median 2.87 pg/mL, range 0.15–7.61 pg/mL) (P=0.0251). No significant difference was found in serum TNF-α, IL-6, IL-8, and IL-1Ra levels before and after LCAP.
Figure 5: Serum cytokines before and after LCAP. To evaluate the effect of postoperative LCAP on host systemic inflammatory response, serum cytokines were measured using blood samples taken before and after LCAP. Serum NE after LCAP was significantly higher than before LCAP (P=0.0251). There was no significant difference in serum TNF-α, IL-6, IL-8, and IL-1Ra levels before and after LCAP.
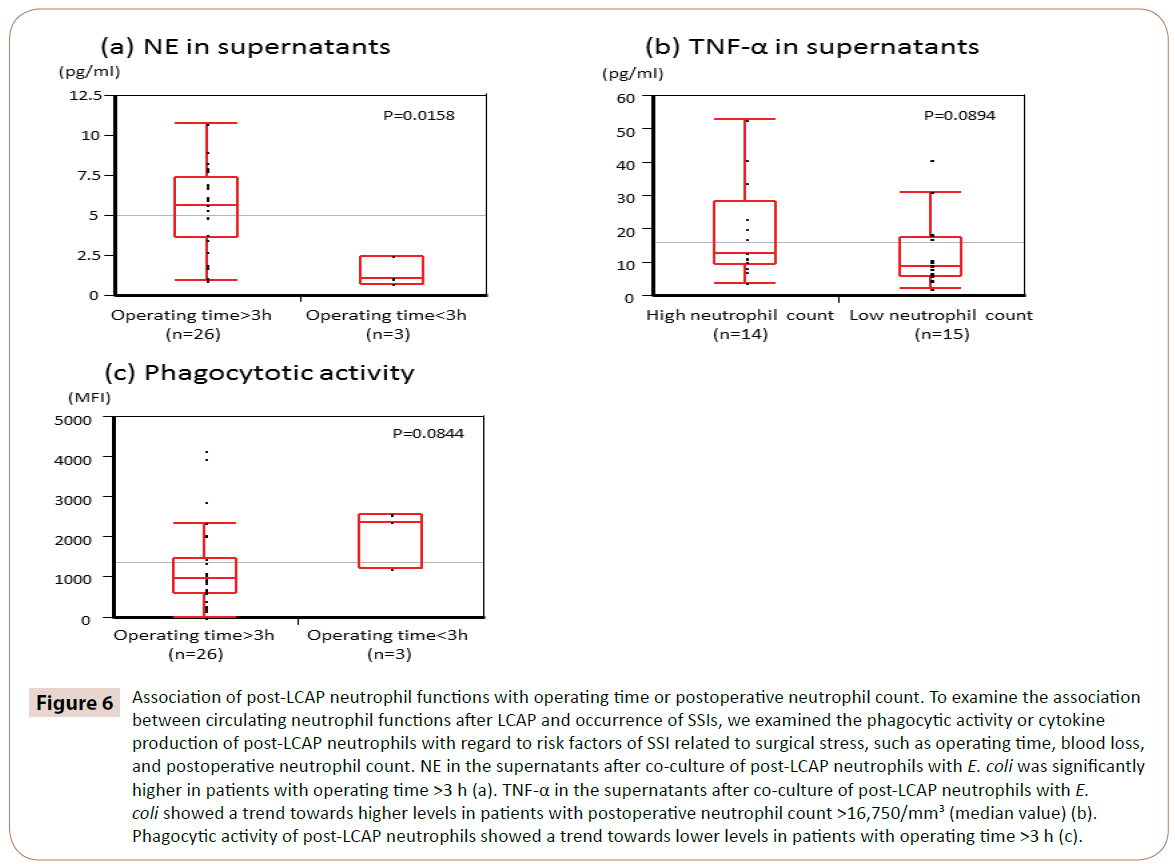
Associations of post-LCAP neutrophil functions with operating time or postoperative neutrophil count
To clarify whether circulating neutrophil functions after LCAP influences the occurrence of SSI, we examined the association between phagocytic activity or cytokine production of post-LCAP neutrophils and risk factors of SSIs related to surgical stress, such as operating time, blood loss, and postoperative neutrophil count.
As shown in Figure 6, NE in the supernatants after co-culture of post-LCAP neutrophils with E. coli was significantly higher in patients with operating time >3 h (median 5.65 pg/mL, range 0.97–10.7 pg/mL) than in those with <3 h operating time (median 1.05 pg/mL, range 0.72–2.44 pg/mL) (P=0.0158).
Figure 6: Association of post-LCAP neutrophil functions with operating time or postoperative neutrophil count. To examine the association between circulating neutrophil functions after LCAP and occurrence of SSIs, we examined the phagocytic activity or cytokine production of post-LCAP neutrophils with regard to risk factors of SSI related to surgical stress, such as operating time, blood loss, and postoperative neutrophil count. NE in the supernatants after co-culture of post-LCAP neutrophils with E. coli was significantly higher in patients with operating time >3 h (a). TNF-α in the supernatants after co-culture of post-LCAP neutrophils with E. coli showed a trend towards higher levels in patients with postoperative neutrophil count >16,750/mm3 (median value) (b). Phagocytic activity of post-LCAP neutrophils showed a trend towards lower levels in patients with operating time >3 h (c).
TNF-α in the supernatants after co-culture of post-LCAP neutrophils with E. coli showed a trend toward higher levels in patients with postoperative neutrophil count >16,750/mm3 (median value) (P=0.0894).
Phagocytic activity of post-LCAP neutrophils against E. coli showed a trend toward lower levels in patients with operating time >3 h (P=0.0844).
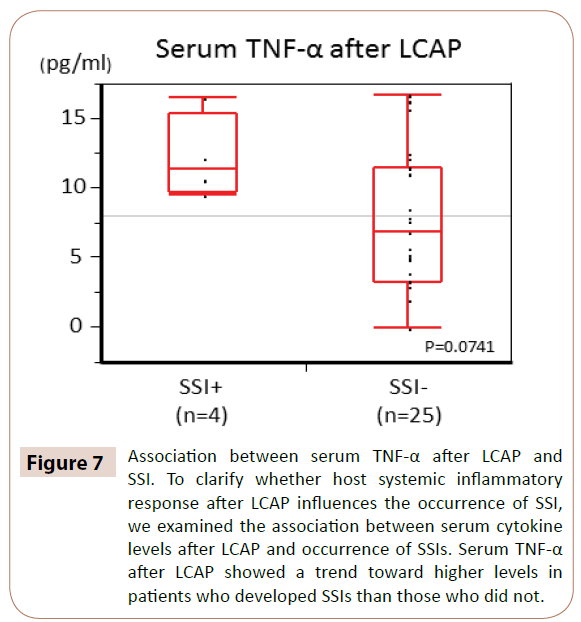
Associations between serum TNF-α after LCAP and SSI
To clarify whether host systemic inflammatory response after LCAP influences the occurrence of SSI, we examined the association between serum cytokine levels after LCAP and occurrence of SSI.
As shown in Figure 7, serum TNF-α after LCAP showed a trend toward higher levels in patients who developed SSIs than in those who did not (P=0.0714).
Figure 7: Association between serum TNF-α after LCAP and SSI. To clarify whether host systemic inflammatory response after LCAP influences the occurrence of SSI, we examined the association between serum cytokine levels after LCAP and occurrence of SSIs. Serum TNF-α after LCAP showed a trend toward higher levels in patients who developed SSIs than those who did not.
There was no significant difference between serum IL-6, IL-8, and IL-1Ra levels after LCAP and occurrence of SSI.
Discussion
Several possible mechanisms of action of LCAP or GMA have been reported [10-13], although their exact mechanisms have not been fully investigated. The variability of results seemed to depend on differences in the blood cells used for analyses (granulocytes, monocytes, or lymphocytes), methods of blood sampling (taken from the inlet or outlet line, or peripheral vein), and the study subjects (healthy volunteers or diseased patients).
Most investigators used leukocytes such as neutrophils, monocytes, or lymphocytes taken from the inlet and outlet lines of an extracorporeal circulation device [11-13]. Leukocytes from the outlet lines are the remaining cells filtered by LCAP. They are returned into the systemic circulation of the patients immediately, and remixed with the circulating leukocytes. Leukocytes from the outlet lines are the small proportion of all circulating leukocytes after LCAP. To clarify the systemic effect of LCAP on the host immune system, we used circulating neutrophils isolated from peripheral veins of UC patients.
Strictly speaking, leukocytapheresis (LCAP) should be termed as leukocytafiltration because it can not only remove leukocytes but also activate them through the filter. In fact, LCAP can’t absorb and remove all blood cells which entered into it. The certain number of blood cells can go through the filter and then return into the circulation. The filtered blood cells are thought to be activated by filtration of LCAP.
Neutrophils are the most abundant population of leukocytes and the most efficiently removed cells by LCAP. The estimated number of filtered leukocytes is nearly 1.1 × 1010 cells per single LCAP session [8, 9], which might be comparable to the removal of nearly half the number of circulating neutrophils (2 × 10–3 × 1010 cells). Thus, we investigated the functional difference between pre-LCAP and post-LCAP neutrophils with regard to the response to E. coli to clarify the potential mechanisms of clinical observation that postoperative LCAP reduced SSI incidence. However, LCAP using Cellsorba can remove and activate not only neutrophils, but also the other blood cells (monocytes, lymphocytes, and platelets) with different removal efficacy. Therefore, it is necessary to investigate the change of the response to E. coli on not only neutrophils, but also the other blood cells between preand post-LCAP, although they were not assessed in this study.
One of the most significant results of the present study was the fact that the percentage of necrotic neutrophils was significantly reduced when post-LCAP neutrophils were co-cultured with E. coli, as compared with pre-LCAP neutrophils. This observation suggests that LCAP decreases the proportion of neutrophils that induce to necrosis by E. coli, and increases the proportion of viable (probably unprimed) neutrophils in the systemic circulation.
Wide variation of the percentage of neutrophil viability, apoptosis, or necrosis in co-cultured with E. coli was observed. In our recent unpublished data, the percentage of early apoptosis (Annexin V+, PI–) in circulating neutrophils ranged widely from approximately 10% to 90% in patients with systemic inflammation such as elevated C-reactive protein >0.3 mg/L. These observations suggest that neutrophils may be already apoptotic without coculture of E. coli due to higher surgical insults.
Necrosis is thought to be uncontrolled cell death, and it seems to release toxic oxygen species and proteolytic enzymes unexpectedly [22, 23]. Neutrophil necrosis is known to be the primary cause of airway and lung damage in the intensely inflamed lungs of patients with cystic fibrosis [24, 25]. Postoperative LCAP may induce the recruitment of a neutrophil subset that is resistant to unexpected or uncontrolled necrotic cell death against bacterial infection into the systemic circulation, in addition to the removal of activated leukocytes that cause systemic inflammation.
In contrast to the above results of ex vivo experiments, serum NE after LCAP was significantly increased. The immediate reduction of serum NE by LCAP was not observed. One possible explanation for this is that a single LCAP session may not suppress sufficiently NE release from whole body neutrophils in surgery-induced systemic inflammation
Circulating neutrophils after LCAP isolated from patients with a longer operating time (>3 h) or higher postoperative neutrophil count (>16,750/mm3; median value) showed not only higher production activity of NE or TNF-α, but also lower phagocytic activity against E. coli. These findings suggest that neutrophils isolated from patients with high surgical stress show excessive production of pro-inflammatory cytokines or impaired phagocytic activity, even after neutrophil renewal by postoperative LCAP.
Among 29 UC patients with postoperative LCAP, only four patients (13.8%) developed SSIs. Their serum TNF-α after LCAP was higher than those without SSIs, although the difference did not reach statistical significance. Therefore, serum TNF-α may be useful for identifying patients who will develop SSI after postoperative LCAP. However, no association between SSIs development and neutrophil viability, apoptosis, or necrosis in co-cultured with E. coli was observed, which may be partly due to small sample size.
Recently, circulating neutrophils have been recognized as a functionally heterogeneous population and differentially primed against various insults [26, 27]. We have reported the clinical significance of circulating neutrophils in systemic inflammation [18-21]. We think that circulating neutrophils may be the primary target to explore the systemic effect of LCAP on the host immune system.
Furthermore, some investigators have reported that leukodepletion, which removes polymorphonuclear cells from the systemic circulation using leukocyte filters, leads to significant improvement in pulmonary and renal function of patients who develop systemic inflammatory response syndrome (SIRS) after cardiopulmonary bypass [28]. Leukodepletion targeting of neutrophils may be beneficial to prevent organ failure due to SIRS [29].
In conclusion, this is believed to be the first report demonstrating that postoperative LCAP decreases the subset of circulating neutrophils that induce necrosis against bacterial infection. It may be beneficial for the prevention of SSI to reduce the unexpected or uncontrolled necrotic cell death caused by the release of toxic or proteolytic substances into the systemic circulation.
Acknowledgement
The authors thank Motoko Ueeda and Chihiro Hibi for providing excellent technical assistance.
Authorship Contribution
K.T., T.A., and M.K. designed and performed the experiments, analyzed and interpreted the data, and wrote the manuscript. S.Y., Y.O., and S.K. performed the experiments and analyzed the data. Y.T., M.I., M.O., M.K., Y.I., K.U., and Y.M. designed the experiments and analyzed and interpreted the data. C.M. and M.K. designed the experiments and interpreted the data.
Grant Support and Disclosure of Financial Arrangements
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI 23791523 to S.Y., 25861181 to Y.O., and 24791887 to M.I.).
References
- Summers C, Rankin SM, Condliffe AM,Singh N, Peters AM, et al. (2010) Neutrophil kinetics in health and disease. Trends Immunol31:318-324.
- Geering B, Stoeckle C, Conus S, Simon HU (2013) Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol34:398-409.
- Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C,et al. (2013) Neutrophils in innate and adaptive immunity. SeminImmunopathol35:377-394.
- Aziz M, Jacob A, Yang WL, Matsuda A, Wang P (2013) Current trends in inflammatory and immunomodulatory mediators in sepsis. J LeukocBiol93:329-342.
- Taylor NJ, Nishtala A, Manakkat Vijay GK,Abeles RD, Auzinger G, et al. (2013) Circulating neutrophil dysfunction in acute liver failure. Hepatology57:1142-1152.
- Fukunaga K, Matsumoto T (2012) Current status and future perspectives of leukocytapheresis for inflammatory bowel disease. J GastroenterolHepatol27:997-1003.
- Nakano R, Iwakiri R, Ikeda Y,Kishi T, Tsuruoka N, et al. (2013) Factors affecting short- and long-term effects of leukocyte removal therapy in active ulcerative colitis. J GastroenterolHepatol28:303-308.
- Ueki Y, Yamasaki S, Kanamoto Y,Kawazu T, Yano M, et al. (2000) Evaluation of filtration leucocytapheresis for use in the treatment of patients with rheumatoid arthritis. Rheumatology (Oxford)39:165-171.
- Shirokaze J (2002) Leukocytapheresis using a leukocyte removal filter. TherApher6: 261-266.
- Shibata H, Kuriyama T, Yamawaki N (2003) Cellsorba. TherApher Dial 7:44-47.
- Hanai H, Iida T, Ikeya K, Abe J, Maruyama Y, et al. (2013) A new paradigm in ulcerative colitis: regulatory T cells are key factor which induces/exacerbates UC through an immune imbalance. MolImmunol54:173-180.
- Kashiwagi N, Sugimura K, Koiwai H, Yamamoto H, Yoshikawa T,et al. (2002)Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci47:1334-1341.
- Ramlow W, Emmrich J, Ahrenholz P, Sparmann G, Kashiwagi N,et al. (2005) In vitro and in vivo evaluation of Adacolumncytapheresis in healthy subjects. J ClinApher20:72-80.
- Korol E, Johnston K, Waser N, FrangiscosSifakis, Hasan S J, et al. (2013)A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS One 8:e83743.
- Araki T, Okita Y, Uchino M, Ikeuchi H, Sasaki I, et al. (2013) Risk factors for surgical site infection in Japanese patients with ulcerative colitis: a multicenter prospective study. Surg Today.
- Miki C, Okita Y, Yoshiyama S,Araki T, Uchida K, et al. (2007)Early postoperative application of extracorporeal leukocyte apheresis in ulcerative colitis patients: results of a pilot trial to prevent postoperative septic complications. J Gastroenterol42:508-509.
- Itabashi M, Ikeuchi H, Araki T, Kono T, Nakamura T, et al. (2008) Effectiveness of leukocytapheresis in suppressing the occurrence of surgical site infections following surgery for ulcerative colitis. Surg Today 38:609-617.
- Miki C, Yoshiyama S, Okita Y, Araki T, Uchida K,et al. (2006) Neutrophil priming as a surgery-related risk factor for postoperative infectious complications in patients with ulcerative colitis. Dig Surg 23:179-185.
- Miki C, Ohmori Y, Yoshiyama S, Toiyama Y, Araki T,et al. (2007) Factors predicting postoperative infectious complications and early induction of inflammatory mediators in ulcerative colitis patients. World J Surg 31:522-529.
- Yoshiyama S, Miki C, Okita Y, Araki T, Uchida K,et al. (2008) Neutrophil-related immunoinflammatory disturbance in steroid-overdosed ulcerative colitis patients. J Gastroenterol43:789-797.
- Okita Y, Miki C, Yoshiyama S,Otake K, Araki T, et al. (2011) Neutrophil dysfunction in steroid-overdosed patients with ulcerative colitis: potential relevance of macrophage migration inhibitory factor to increased postoperative morbidity. Surg Today 41:1504-1511.
- Kroemer G, Galluzzi L, Vandenabeele P, J Abrams, ES Alnemri, et al. (2009) Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3-11.
- van den Berg CW, Tambourgi DV, Clark HW, Hoong SJ, Spiller OB, et al. (2014)Mechanism of neutrophil dysfunction: neutrophil serine proteases cleave and inactivate the C5a receptor. J Immunol192:1787-1795.
- Rydell-Törmänen K, Uller L, Erjefält JS (2006)Direct evidence of secondary necrosis of neutrophils during intense lung inflammation. EurRespir J 28:268-274.
- TsaoFH, Xiang Z, Abbasi A, Meyer KC (2012) Neutrophil necrosis and annexin 1 degradation associated with airway inflammation in lung transplant recipients with cystic fibrosis. BMC Pulm Med 12:44.
- Pillay J, Ramakers BP, Kamp VM,Hoong SJ, Spiller OB, et al. (2010) Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J LeukocBiol88:211-220.
- Mócsai A (2013)Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 210:1283-1299.
- Treacher DF, Sabbato M, Brown KA, Gant V (2001)The effects of leucodepletion in patients who develop the systemic inflammatory response syndrome following cardiopulmonary bypass. Perfusion. 16:67-73.
- Lewis SM, Khan N, Beale R, Treacher DF, Brown KA (2013) Depletion of blood neutrophils from patients with sepsis: treatment for the future? IntImmunopharmacol17:1226-1232.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences