Groove Pancreatitis: A Description of Four Cases and Literature Review
Díaz-Jaime FC, Herreras-Lopez J, Sáez-Gonzalez E, Higón-Ballester MD, Torregrosa-Andrés A , Pareja-Ibars E, Orbis-Castellanos F and Moya-Herraiz A
DOI10.4172/2472-1891.100012
Díaz-Jaime FC1*, Herreras-Lopez J1, Sáez-Gonzalez E1, Higón-Ballester MD1, Torregrosa-Andrés A2 , Pareja-Ibars E3, Orbis-Castellanos F3 and Moya-Herraiz A3
1Digestive Medicine Department, University Hospital La Fe, Valencia, Spain.
2Radiology Department, University Hospital La Fe, Spain.
3General Surgery Department, University Hospital La Fe, Valencia, Spain.
- *Corresponding Author:
- Francia Díaz
Digestive medicine Department, University Hospital La Fe
Avda Fernando Abril Martorell 106, Valencia 46026, Spain
E-mail: francia_diaz2003@hotmail.com
Received date: November 04, 2015; Accepted date: December 21 2015; Published date: December 28, 2015
Citation: Díaz-Jaime FC,Herreras-Lopez J, Sáez-Gonzalez E, et al. Groove Pancreatitis: A Description of Four Cases and Literature Review . Int J Dig Dis. 2016, 2:1. doi:10.4172/2472-1891.100012
Abstract
Groove pancreatitis is a rare form of chronic pancreatitis, affecting the area between the duodenum, common bile duct and pancreatic head. The pathophysiology of groove pancreatitis is unclear, and several mechanisms have been proposed. This disease can mimic duodenal, pancreatic or periampular neoplasms. We present a series of 4 patients diagnosed of groove pancreatitis at a tertiary medical center over a 3-year period. Three of the patients were surgically treated, recovering weight and removing pain. The other one was treated with medical treatment. Same results were observed. Brief review of the literature was performed.
Keywords
Groove pancreatitis; Paraduodenal pancreatitis; Paraduodenal wall cyst; Cystic dystrophy of heterotopic pancreas
Abbreviations
GP: Groove Pancreatitis; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; EUS: Endoscopic Ultrasonography; ERCP: Endoscopic Retrograde Cholangiopancrea¬tography; CDP: Cephalic Duodenopancreatectomy
Introduction
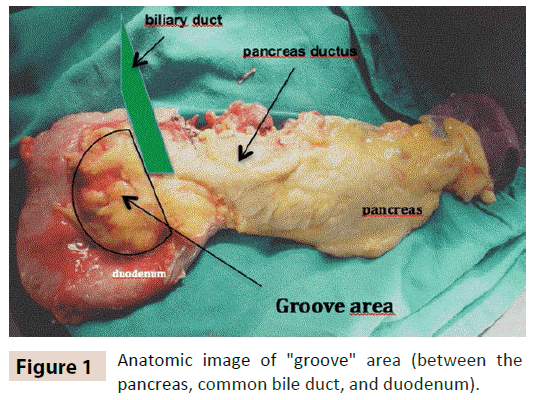
Groove pancreatitis (GP) is usually a segmental chronic pancreatitis, characterized by fibrotic inflammation of the “groove” between the head of the pancreas, duodenum and common bile duct (figure 1). [1, 2] It is considered to be infrequent maybe due to an under diagnosis. The real prevalence is unknown and some studies report a prevalence as high as 24.5% in pancreaticoduodenectomy specimens from patients with chronic pancreatitis [3].
GP has received different names in the literature including paraduodenal wall cyst, pancreatic hamartoma of the duodenum, cystic dystrophy of heterotopic pancreas and myoadenomatosis. The different terms used to describe this clinical entity makes it difficult to have accurate bibliographic information [4].
GP occurs mainly in alcoholicand/or smoking men in the fifth decade of life. The predominant symptoms in these patients are upper abdominal pain and recurrent vomiting mainly due to duodenal stenosis often leading to significant weight loss. Sometimes there is jaundice too. Imaging typically reveals duodenal stenosis and cystic lesion(s) near the head of the pancreas [5].
This condition can often be difficult to differentiate from pancreatic head adenocarcinoma but certain clinical, radiological and histological features can be useful in making a diagnosis. When the diagnosis is clear, GP can be treated by conservative medical measures, including endoscopic therapy as the first line of intervention. [6] However, surgery is often required when clinical symptoms are severe and not responding to medical or conservative treatment as well as to rule out malignancy. We provide a summary of 4 patient diagnosed of groove pancreatitis.
Case report
Four cases of groove pancreatitis were identified over a 3-year period. The 4 cases were diagnosed based on the clinical presentation, imaging findings and histological confirmation of GP. Table 1 shows a summary of the findings for each case. All patients were men, with an average age of 37 years old. Significant alcohol abuse (80-100 gr/day) history was confirmed in three of the four patients and all of them were active smokers (about 20 packs years).
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age | 36 | 37 | 30 | 45 |
| Sex | Male | Male | Male | Male |
| Alcohol | Yes | No | Yes | Yes |
| Tobacco | Yes | Yes | Yes | Yes |
| Symptoms | Vomiting, abdominal pain, weight loss | Vomiting, abdominal pain, weight loss, jaundice. | Vomiting, abdominal pain, weight loss | Vomiting, abdominal pain, weight loss, jaundice |
| CT | Edema and thickening with several cyst on the duodenal wall. Calcification in the pancreatic head | Hipoenhancing focus in the pancreatic head. Edema and thickening with several cyst on the duodenal wall Peripancreatic collection | Edema and thickening with several cyst on the duodenal wall. Peripancreatic collection | Low density mass in the groove area. Edema and thickening with several cyst on the duodenal wall |
| MRI | T1 hipointense image and hyperintese in T2 pancreas. Cysts in duodenum’s wall with stenosis | Pancreatic head and duodenum lesion mass. T1 hipointense image and hyperintese in T2. Cysts in duodenum’s wall with stenosis | T1 hipointense image and hyperintese T2 in pancreas. Cysts in duodenum’s wall with stenosis | Pancreatic head and duodenum mass. T1 hipointense image and hyperintese in T2. Cysts in duodenum’s wall with stenosis |
| EUS | Some cysts located in the second part of duodenum’s wall | Chronic pancreatitis. Some anechogenous images in the duodenum’s wall | Some cysts located in the second part of duodenum’s wall | Chronic pancratitis. Cysts inside the muscularispropria and thinckening of the duodenal wall |
| Pathology | Hyperplasia of Bruner’s gland. Extensive fibrosis and some inflammation in duodenum’s wall | Hyperplasia of Bruner’s gland Extensive edema, inflammation in duodenum’s wall. Without atypias |
Hyperplasia of Bruner’s gland. Fibrosis, edema and necrosis in pancreas and duodenum. No atypias |
---- |
| Treatment | CDP | CDP | CDP | Medical |
| CT: Computed tomography; CDP: Cephalic duodenopancratectomy | ||||
Table 1: Clinical, radiological and histopathological characteristics of patients with groove pancreatitis.
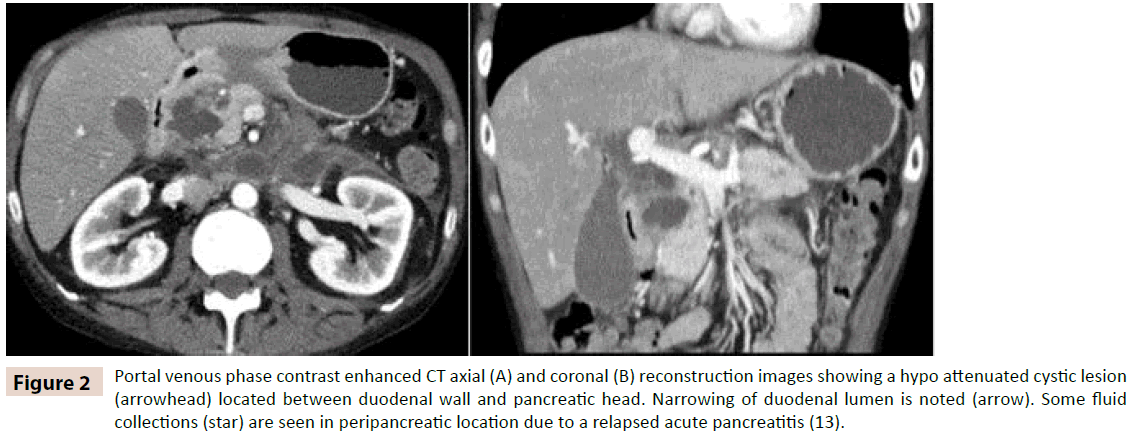
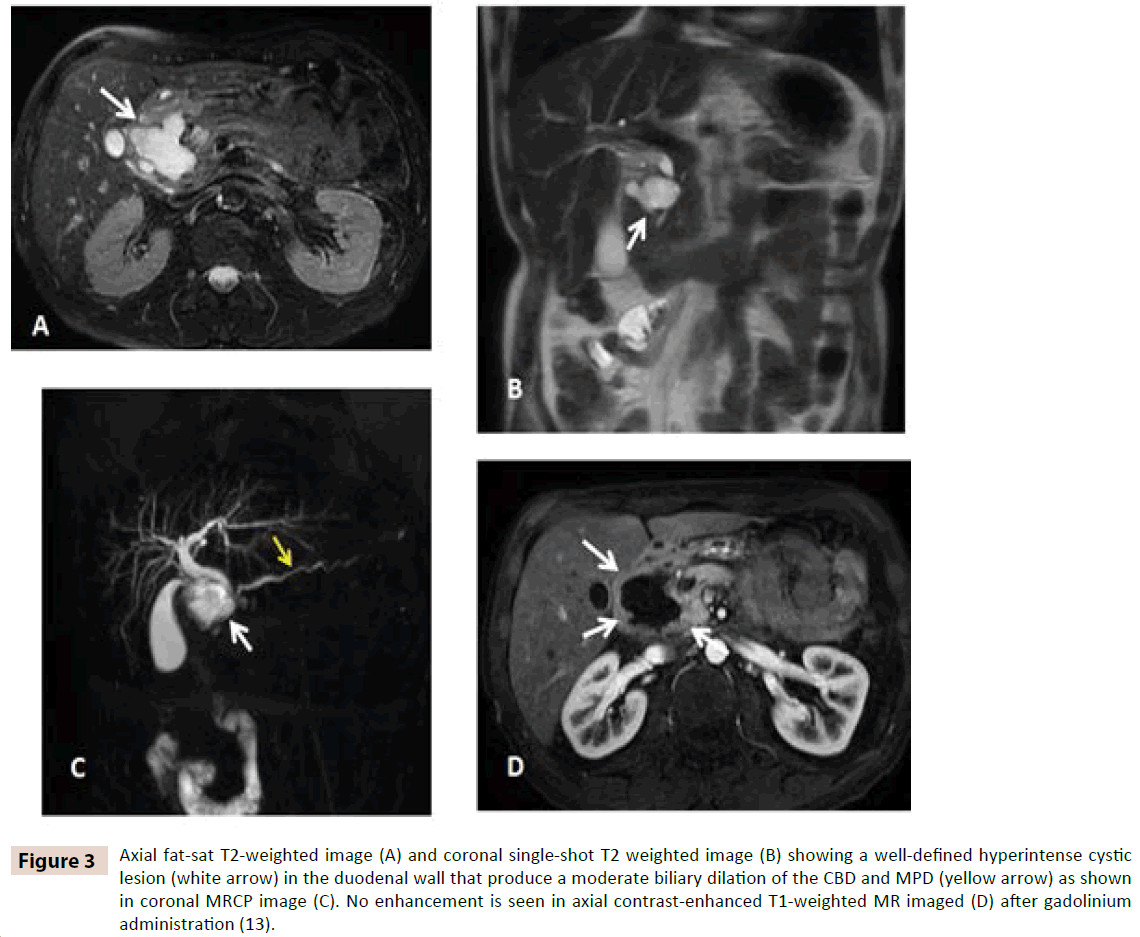
Clinical presentation was abdominal pain, vomiting and weight loss. Two patients had high level of bilirubin (2,62 mg/dl and 6,7 mg/dl) and cholestasis enzymes (GOT 654 and 82 U/l, GPT 769 and 60 U/l, GGT 890 and 1044 U/l), amylase (280 and 230 mg/dl), negative tumors markers in both patients. Computed tomography (CT) (figure 2A and 2B) and magnetic resonance imaging (MRI) (figure 3A, 3B, 3C and 3D) was performed in every case revealing a mass lesion occupying the pancreaticoduodenal groove in two patients. Edema and thickening with several cysts on the duodenal wall was found in all cases. In two of the patients a peripancreatic collection was discovered.
Figure 2: Portal venous phase contrast enhanced CT axial (A) and coronal (B) reconstruction images showing a hypo attenuated cystic lesion (arrowhead) located between duodenal wall and pancreatic head. Narrowing of duodenal lumen is noted (arrow). Some fluid collections (star) are seen in peripancreatic location due to a relapsed acute pancreatitis (13).
Figure 3: Axial fat-sat T2-weighted image (A) and coronal single-shot T2 weighted image (B) showing a well-defined hyperintense cystic lesion (white arrow) in the duodenal wall that produce a moderate biliary dilation of the CBD and MPD (yellow arrow) as shown in coronal MRCP image (C). No enhancement is seen in axial contrast-enhanced T1-weighted MR imaged (D) after gadolinium administration (13).
All patients underwent upper digestive endoscopy, demonstrating edema and non-obstructive stenosis of the duodenal lumen.
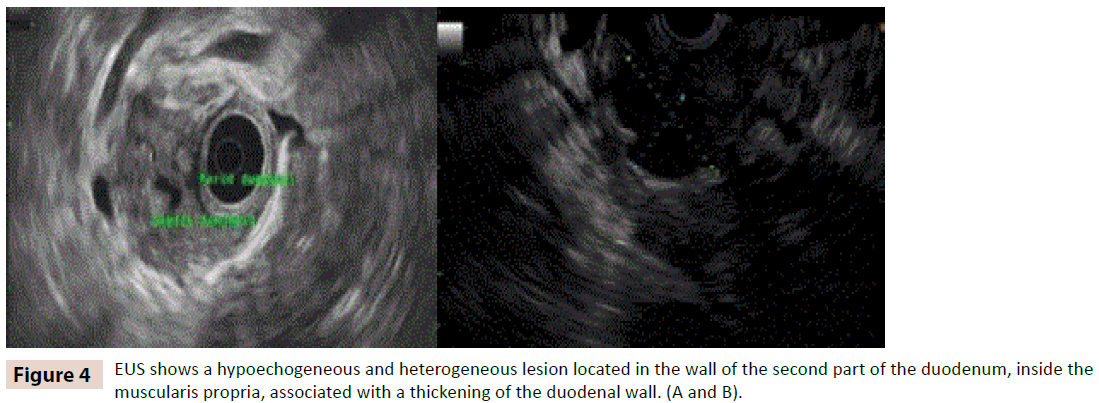
Endoscopic ultrasound (EUS) was performed in the four patients and presence of multiple cysts in the duodenal wall was confirmed in all cases (figure 4A and 4B). Chronic pancreatitis was noted in three cases, all of them alcohol related. The other case with no alcohol history at onset of disease was an acute pancreatitis. Malignant cells were not identified by fine needle aspiration in any case. Finally three patients required surgical treatment with cephalic duodenopancreatectomy (CDP) due to untreatable pain and duodenal stenosis which produced oral intolerance. Only one patient continues with medical treatment (opioid analgesics and endoscopic drainage). Two months later of medical treatment the patient is asymptomatic without abdominal pain, nausea or vomiting. He has improved his weight, but he is still smoking.
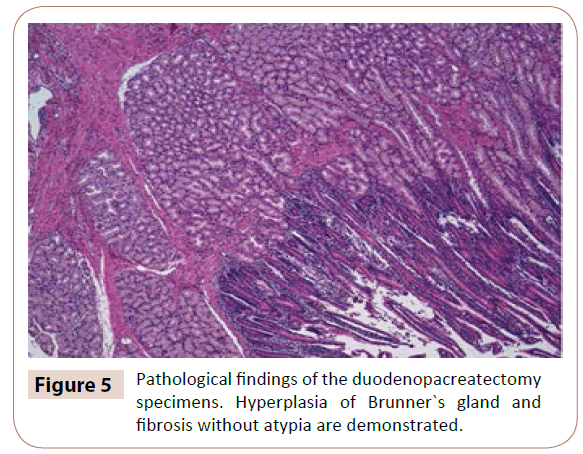
The pathological findings of the duodenopacreatectomy specimens confirmed ductal ectasia with interior proteic and eosinophilic content, associated with multifocal hyperplasia of Brunner`s gland and fibrosis without atypia (figure 5).
Discussion
Knowledge of the clinical and pathological features of groove pancreatitis is important because it allows a preoperative diagnosis that in most cases correctly differentiates it from pancreatic and periampullary neoplasms and may prevent unnecessary surgical resection [6]
Etiology of GP is heterogeneous with a variety of factors playing a role in its development, including: anatomic disruption or functional obstruction of the minor papilla, disruption of the pancreatic juice flow, pancreatic heterotopia in the duodenum, pancreas divisum, chronic alcohol consumption and secondary modifications of local anatomy due to gastrectomy, gastroduodenal ulcer and biliary disease [7,8,9].
GP usually exhibits pain in the upper abdomen, associated with recurrent vomiting and weight loss due to duodenal obstruction. [6] Some cases present diarrhoea, mellitus diabetes and more infrequently fluctuant jaundice. [5] Its course is usually chronic and there are some reports of perforation, gastrointestinal bleeding and malignant degeneration of the heterotopic pancreas as complications. [10] Preoperative diagnostic of GP is often difficult to establish. Ultrasound typically shows a hypoechoic mass with thickening of the duodenal wall. CT has more accuracy and the findings are characterized by a hypodense, hypo enhancing pancreatic head mass and a thickened duodenal wall. The most characteristic MRI finding is a sheet-like mass between the head of the pancreas and the duodenum, hypointense in T1 and hyper-iso-hipointense in T2 (by stage), associated with duodenal wall cystic changes and thickening. Endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasonography (EUS) are often useful in the differentiation of groove pancreatitis from pancreatic adenocarcinoma and should be used early in evaluation even when cross-sectional imaging is not suggestive of a mass. [10]
Abstinence from alcohol and tobacco, rest, and opioid analgesics are the most frequently used conservative measures. When the symptoms do not improve after medical treatment, surgery (CDP) is the best option according to scientific evidence and in our experience. [7] However, it has been demonstrated in recent publications that only in some patients with untreatable symptoms, a stepwise approach to treatment of GP is feasible. But actually the real efficacy is not known. In a large retrospective case series using endoscopic approach, associated with medical treatment, a complete clinical success in nearly 70% of patients was reached in five years. Nevertheless, prospective, controlled studies are needed to confirm these findings [6].
Conclusion
GP is a rare disease that can mimic duodenal, pancreatic or periampular neoplasms. Awareness of this condition should be kept in mind when making the differential diagnosis between pancreatic masses, duodenal stenosis and/or cysts. Correct preoperative identification depends on knowledge of clinical, radiologic, and cytologic features. In the treatment of GP, a medical with or without endoscopic treatment should be tried before surgery. Currently, surgery treatment remains the gold standard (CDP) in achieving weight recovery and disappearance of pain in the majority of patients. (11,12).
References
- Becker V, Mischke U (1991) Groove pancreatitis. Int J Pancreatol 10:173-182
- Stolte M, Weiss W, Volkholz H, Rösch W (1982) A special form of segmental pancreatitis: Groove pancreatitis. Hepatogastroenterology 29: 198?208.
- Balakrishnan V, Chatni S, Radhakrishnan L, Narayanan VA, Nair P (2007) Groove pancreatitis: a case report and review of literature. JOP8: 592-597.
- Adsay NV, Zamboni G (2004) Paraduodenal pancreatitis: a clinicopathologically distinct entity unifying ?cystic dystrophy of heterotopic pancreas?, ?para-duodenal wall cyst?, and ?groove pancreatitis?. SeminDiagnPathol 21:247-254.
- Rebours, V, Lévy P, Vullierme MP, Couvelard A, O'Toole D, et al. (2007) Clinical and morphological features of duodenal cystic dystrophy in heterotopic pancreas. The Am J gastroenterol 102: 871-879.
- Arvanitakis M, Rigaux J, Toussaint E, Eisendrath P, Bali MA, et al. (2014) Endotherapy for paraduodenal pancreatitis: a large retrospective case series. Endoscopy 46: 580-587.
- DeSouza K, Nodit L (2015) Groove pancreatitis: a brief review of a diagnostic challenge. Arch Pathol Lab Med 139: 417-421.
- Pezzilli R, Santini D, Calculli L, Casadei R, Morselli-Labate A (2011) Cystic dystrophy of the duodenal wall is not always associated with chronic pancreatitis. WJG, 17: 4349.
- Black TP, Guy CD, White RR, Obando J, Burbridge RA (2014) Groove pancreatitis: four cases from a single center and brief review of the literature. ACG case reports journal 1: 154.
- Pallisera A, Ramia JM, Vicens C, Cifuentes A (2015) Groove pancreatitis. Rev EspEnferm Dig 107: 280- 288.
- Jounnaud V, Coutarel P, Tossou H, Butel J, Vitte R, et al. (2006) Cystic distrophy of the duodenal wall associated with chronic alcoholic pancreatitis. GastroenterolClinBiol30:580-586.
- Rahman SH, Verbeke CS, Gomez D, McMahon MJ, Menon KV (2007)Pancreatico-duodenectomy for complicated groove pancreatitis. HPB (Oxford) 9: 229?234.
- Hungerford JP, NeillMagarik MA, Hardie AD (2015)Thebreadth of imagingfindings of Groove pancreatitis. ClinImaging39:363-366.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences