Patient Satisfaction with IBS Symptom Relief Using a Novel Peppermint Oil Delivery System in a Randomized Clinical Trial and in the General Population
Cash BD, Epstein MS and Shah SM
DOI10.4172/2472-1891.100027
Cash BD1*, Epstein MS2 and Shah SM3
1Division of Gastroenterology, University of South Alabama, Mobile, AL, USA
2Digestive Disorders Associates, Annapolis, MD, USA
3IM Health Science LLC, Boca Raton, FL, USA
- *Corresponding Author:
- Cash BD
Gastroenterology Division, University of
South Alabama, 6000 University Commons
75 University Blvd, Mobile, AL 36688, USA
Tel: 251-660-5555
Fax: 251-660-5559
E-mail: cash@health.southalabama.edu
Received date: March 14, 2016; Accepted date: April 19, 2016; Published date: April 22, 2016
Citation: Cash BD, Epstein MS, Shah SM. Peppermint Oil Delivery System in a Randomized Clinical Trial and in the General Population. Int J Dig Dis. 2016, 2:2. doi:10.4172/2472-1891.100027
Abstract
Background: A recent randomized clinical trial (RCT) of a novel peppermint oil preparation (PO-SST) demonstrated significant relief of irritable bowel syndrome (IBS) symptoms versus placebo. In addition to efficacy, patient satisfaction and post-marketing utilization are also important to assess.
Methods: The RCT included an end-of-study questionnaire for subjects in the active arm to evaluate overall satisfaction with IBS symptom relief. A separate post-marketing study was conducted in the general population to assess real world satisfaction and dosing frequency of open label PO-SST in patients with IBS. The dosing frequency in the RCT was fixed at 2 capsules three times daily (TID), while the post-marketing patient population was allowed flexible dosing.
Results: Thirty-five subjects randomized to PO-SST in the RCT and 285 patients in the post-marketing study were included in this analysis. There was a high satisfaction rate (>80%) with PO-SST in both studies. Most patients in the postmarketing population (60.8%) used 1 to 2 capsules per day, and 75.6% reported IBS symptom reduction within 1-2 hours of ingestion. Patients in the RCT reported significant reduction in IBS symptoms 24 hours after the first dose of PO-SST.
Discussion: PO-SST showed a high rate of satisfaction among patients participating in an RCT and the general patient population. Symptom improvement was rapid and remarkably similar in both groups, despite the lower daily capsule intake by patients in the general population.
Keywords
IBS; Peppermint oil; Patient satisfaction; Outcome; Quality of life
Abbreviations
BM: Bowel Movement; IBS: Irritable Bowel Syndrome; IBSACT: IBS Adherence and Compliance Trial; IBS-C: Constipation-predominant IBS; IBS-D: Diarrhoea-predominant IBS; IBS-M: Mixed IBS; IBSREST: Irritable Bowel Syndrome Reduction Evaluation and Safety Trial; PO: Peppermint Oil; PO-SST: Peppermint Oil with Site Specific Technology; RCT: Randomized Controlled Trial; TID: Three Times Daily
Summary Box
What is already known?
• Peppermint oil can be an effective therapy for IBS.
• Some versions of peppermint oil are poorly tolerated due to GERD symptoms and dyspepsia.
What is new?
• Patients with IBS in a randomized clinical trial and in the general population reported consistently high overall satisfaction with relief in IBS symptoms and quality of life while taking a novel peppermint oil formulation.
• Peppermint oil designed with site specific targeting may be a valued option for patients who desire rapid relief of IBS symptoms.
How might these findings impact clinical practice?
• Increase the willingness to use peppermint oil as an ondemand therapy for IBS related symptoms.
• Facilitate additional evaluation of the mechanism of action of peppermint oil in IBS patients.
Introduction
Irritable bowel syndrome (IBS) is a chronic functional bowel disorder with periodic exacerbations of multiple symptoms, including abdominal pain or discomfort, constipation, diarrhea, abdominal bloating, a sensation of incomplete evacuation, urgency of bowel movement (BM), pain at evacuation, and passage of gas or mucus [1,2]. According to the Rome III criteria, IBS can be subtyped based on predominant bowel habit patterns into mixed IBS (IBS-M), diarrhea-predominant IBS (IBS-D), and constipation-predominant IBS (IBS-C) [3].
Patients with IBS often have symptoms that are severe enough to impair quality of life, result in significant utilization of healthcare resources, cause high rates of absenteeism from work and school, [4] and endorse a high rate of dissatisfaction with the treatments they receive for their symptoms [5]. Furthermore, limited options are available for patients with IBS to obtain rapid relief during symptomatic flares. L-menthol, the primary active component of peppermint oil (PO), has been shown to possess anti-spasmodic, anti-serotonergic, anti-inflammatory, anti-bacterial, kappa opioid agonist, and carminative properties, making PO an attractive therapy for IBS [6-11]. However, unreliable delivery with single-unit, enteric-coated, liquid-filled PO capsules has limited their benefit—especially with heartburn and dyspepsia side effects [12].
The Irritable Bowel Syndrome Reduction Evaluation and Safety Trial (IBSREST), a 4-week, placebo controlled, randomized clinical trial (RCT), evaluated the efficacy of a novel PO formulation utilizing site specific technology (PO-SST) involving solid state, triple-coated microspheres of highly purified PO designed to provide rapid, reliable, and sustained release of PO in the small intestine [13]. Patients receiving PO-SST experienced significant relief of multiple individual IBS symptoms as well as improvement in symptoms rated as severe or unbearable compared with placebo, without significant adverse events observed with previous PO preparations [13]. In addition to individual symptom improvement, patient satisfaction with therapy was an important endpoint in IBSREST that has not been previously reported. The trial was conducted at 4 geographically diverse study sites in the United States, in accordance with good clinical practice and applicable regulatory requirements and ethical principles. The protocol was approved by the Chesapeake Institutional Review Board and the Palm Beach Clinical Research Organization (West Palm Beach, FL, USA) was responsible for conduct of the study.
A separate post-marketing evaluation of open label PO-SST,the IBS Adherence and Compliance Trial (IBSACT), was also conducted in order to determine real-world daily PO-SST capsule usage frequency and patient satisfaction in a general population of patients with IBS. Herein, we report the overall satisfaction with relief of IBS symptoms for patients in both studies as well as the real-world daily PO-SST capsule intake observed in the IBSACT.
Methods
Study subjects
The details of the IBSREST have been previously published.(13) Briefly, patients met Rome III criteria for IBS-M or IBS-D, had average daily IBS-related abdominal pain of ≥ 4 (0-10 scale), a Total IBS Symptom Score of ≥ 2 (0-4 scale), and were 18-60 years of age. Subjects had to conÃÆïÃâìÃâÃÂrm that they were not planning to change their usual diet and lifestyle during the study. Patients with a diagnosis of IBS-C or IBS-unubtyped as deÃÆïÃâìÃâÃÂned by the Rome III criteria or a history of inÃÆïÃâìÃâââ¬Å¡ammatory or immune-mediated gastrointestinal disorders were excluded from the IBSREST. The protocol did not allow concomitant or rescue medications during the trial. The IBSACT included patients who were using openlabel PO-SST (marketed as IBgard®) in the general population of patients with IBS. There were no restrictions on concomitant medications for patients participating in the IBSACT. The POSST formulations were identical in each study and PO-SST was indistinguishable from placebo in the IBSREST trial.
Experimental design
Patients in the IBSREST were randomized to receive either PO-SST (2 capsules, each containing 90 mg of PO) or identical placebo three times daily (TID) for 4 weeks. A 7 question endof- study questionnaire (Table 1) was utilized to evaluate overall satisfaction with the relief of IBS symptoms for all subjects in the IBSREST. Patients completed the written questionnaire during their last study visit, prior to unblinding. Counts and percentages of subjects with each response were tabulated only for subjects randomized to the PO-SST treatment group.
| Question | Response Choices |
|---|---|
| Overall, how would you rate the improvement in your IBS symptoms during the treatment period? | • No Improvement • Moderate Improvement • Major Improvement |
| Overall, how would you rate the improvement in your quality of life during the treatment period? | • No Improvement • Moderate Improvement • Major Improvement |
| Overall, how would you rate the improvement in your abdominal pain/discomfort during the treatment period? | • No Improvement • Moderate Improvement • Major Improvement |
| Overall, how would you rate the improvement in your bowel regularity? | • No Improvement • Moderate Improvement • Major Improvement |
| How likely are you to recommend the study product to family or friends who have Irritable Bowel Syndrome (IBS)? | • Not Likely • Likely • Very Likely |
| If given the option, how likely is it that you would continue taking the study product? | • Not Likely • Likely • Very Likely |
| Would you be willing to be contacted in the future about the study product? | • Yes • No |
Table 1: IBSREST post-dose patient satisfaction questionnaire.
Patients in the IBSACT were allowed flexible dosing of PO-SST. A 10 question survey (Table 2) was conducted with patients who were using PO-SST in the general IBS patient population to assess patient satisfaction and dosing frequency in the realworld setting. The study questionnaire was included in PO-SST physician samples, giving all patients receiving PO-SST samples from their physician the opportunity to fill out the questionnaire. Patients submitted survey responses online or via fax.
| Question | Response Choices |
|---|---|
| How often do you suffer from Irritable Bowel Syndrome (IBS)? | • Daily • Once a week • Twice a week or more • Once a month |
| On average, how many capsules of IBgard® are you taking daily? | • 1-2 • 3-4 • 5-8 • I don't take it daily • Other (please specify) |
| When do you typically take IBgard®? | • Before meals • After meals • Only when I have symptoms • Other (please specify) |
| How long does it take for you to feel relief from your abdominal pain, discomfort and/or bloating after you have taken IBgard®? | • Less than 1 hour • 1-2 hours • 3-8 hours • 8-24 hours • Longer |
| Overall, how would you rate the improvement in your IBS symptoms while taking IBgard®? | • Major improvement • Moderate improvement • No improvement |
| Overall, how would you rate the improvement in your quality of life while taking IBgard®? | • Major improvement • Moderate improvement • No improvement |
| Overall, how would you rate the improvement in your abdominal pain and cramping while taking IBgard®? | • Major improvement • Moderate improvement • No improvement |
| Overall, how would you rate the improvement in your bowel regularity while taking IBgard®? | • Major improvement • Moderate improvement • No improvement |
| How likely are you to recommend IBgard® to family or friends who have Irritable Bowel Syndrome (IBS)? | • Very likely • Likely • Not likely |
| How likely is it that you will continue taking IBgard® for your IBS? | • Very likely • Likely • Not likely |
Table 2: IBSACT post-marketing patient satisfaction survey.
Patient perception of change in quality of life after receiving PO-SST was assessed in both the IBSREST and IBSACT by asking the question, “Overall, how would you rate the improvement in your quality of life during the treatment period?” Patients were given three response options to choose from: no improvement, moderate improvement, or major improvement.
Results
Patients
Thirty-five subjects with moderate to severe IBS randomized to PO-SST in IBSREST completed the end-of-study questionnaire to evaluate overall satisfaction with IBS symptom relief. Sixteen of these patients had IBS-M and 19 patients had IBS-D. Demographics for this population have been reported previously [13].
From the general population, 285 patients with IBS, who had received PO-SST samples from their physicians, completed the IBSACT survey. Demographic information was not collected with the IBSACT survey. The majority of patients in IBSACT reported suffering from IBS symptoms daily (62.9%) or twice a week or more (23%).
Dosage frequency
In the IBSREST, the dosage of PO-SST was fixed per protocol at 2 capsules TID. The dosage frequency reported by the general population of patients with IBS surveyed in the IBSACT was considerably lower with 60.8% of patients using only 1 to 2 capsules per day (Table 3). More than half (55.2%) of patients in the IBSACT took PO-SST before meals and 24.9% took PO-SST after meals.
| Average number of PO SST capsules taken daily | IBSREST (n=35) | IBSACT (n=285) |
|---|---|---|
| 5-8 | 100% (6 per day) | 4.6% |
| 3-4 | N/A | 26.5% |
| 1-2 | N/A | 60.8% |
| Not taken Daily | N/A | 4.6% |
| Other | N/A | 3.5% |
Table 3: Dosing of PO-SST capsules.
Timing of symptom relief
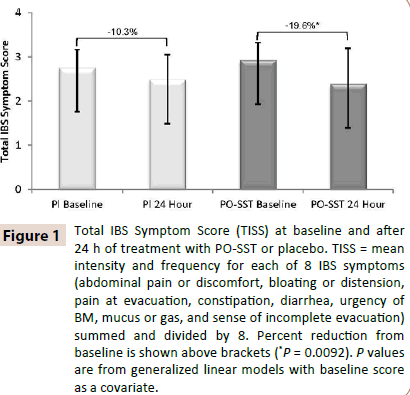
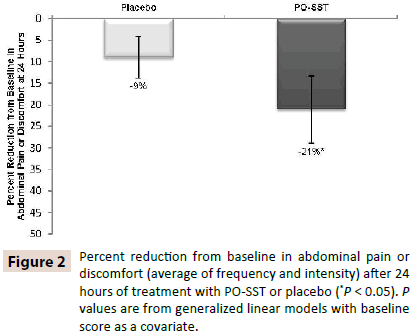
Patients in the active arm of the IBSREST had a statistically significant reduction versus placebo in total IBS symptom score (Figure 1) and abdominal pain (Figure 2) at the first follow-up assessment, 24 hours after their first dose [13]. IBS symptom reduction occurred within 1-2 hours in 75.6% of patients surveyed in the IBSACT (Table 4).
Figure 1: Total IBS Symptom Score (TISS) at baseline and after 24 h of treatment with PO-SST or placebo. TISS = mean intensity and frequency for each of 8 IBS symptoms (abdominal pain or discomfort, bloating or distension, pain at evacuation, constipation, diarrhea, urgency of BM, mucus or gas, and sense of incomplete evacuation) summed and divided by 8. Percent reduction from baseline is shown above brackets (*P = 0.0092). P values are from generalized linear models with baseline score as a covariate.
| IBSACT (n=285) | |
|---|---|
| Number of hours before experiencing relief | Percentage of patients |
| < 1 hour | 33.7 |
| 1-2 hours | 41.9 |
| 3-8 hours | 14.4 |
| 8-24 hours | 5.2 |
| > 24 hours | 4.8 |
Table 4: Timing of symptom relief in the general IBS population (IBSACT) with open-label PO-SST.
Patient satisfaction
Responses for the surveys in the IBSREST and IBSACT are shown in Tables 3 and 4. When asked to rate their overall improvement in IBS symptoms while taking PO-SST, 98.1% of patients in the IBSACT reported moderate or major improvement, while 88.3% of patients randomized to PO-SST in the IBSREST noted moderateor major improvement. The majority of patients also reported moderate or major improvement in response to the question, “Overall, how would you rate the improvement in your quality of life during the treatment period?” while taking PO-SST (88.3% in IBSREST, 92.7% in IBSACT).
For individual IBS symptoms, 85.3% of patients taking PO-SST in the IBSREST and 95.8% in the IBSACT indicated moderate or major overall improvement in abdominal pain and cramping (Table 5). In addition, 82.3% of patients in the IBSREST and 84.6% of patients in the IBSACT reported a moderate or major overall improvement in bowel regularity while taking PO-SST.
| Major Improvement | Moderate Improvement | No Improvement | ||||
|---|---|---|---|---|---|---|
| IBSREST | IBSACT | IBSREST | IBSACT | IBSREST | IBSACT | |
| Overall improvement in IBS symptoms while taking PO SST | 41.2% | 40.5% | 47.1% | 57.6% | 11.8% | 1.9% |
| Overall improvement in quality of life while taking PO SST | 32.4% | 36.5% | 55.9% | 56.2% | 11.8% | 7.3% |
| Overall improvement in abdominal pain and cramping while taking PO SST | 35.3% | 47.5% | 50.0% | 48.3% | 14.7% | 4.2% |
| Overall improvement in bowel regularity while taking PO SST | 38.2% | 29.8% | 44.1% | 54.8% | 17.6% | 15.4% |
Table 5: Improvement in symptoms and perceived quality of life with PO-SST.
When asked how likely they would be to recommend PO-SST to family or friends who have IBS symptoms, 94.1% of patients in the IBSREST and 97.0% of patients in the IBSACT indicated that they were likely or very likely to recommend PO-SST (Table 6). The majority of patients also reported that they were likely or very likely to continue taking PO-SST for their IBS symptoms (88.3% in IBSREST, 99.3% in IBSACT). In the case of patients from the IBSREST, these recommendations occurred prior to drug unblinding.
| Very Likely | Likely | Not Likely | ||||
|---|---|---|---|---|---|---|
| IBSREST | IBSACT | IBSREST | IBSACT | IBSREST | IBSACT | |
| How likely are you to recommend PO SST to family or friends who have IBS? | 76.5% | 60.5% | 17.6% | 36.5% | 5.9% | 3.0% |
| How likely is it that you will continue taking PO SST for your IBS? | 61.8% | 66.3% | 26.5% | 33.0% | 11.8% | 0.7% |
*IBSREST (blinded); IBSACT (open-label)
Table 6: Recommendation and continuation of PO-SST*.
Discussion
This analysis found that patients reported a consistently high level of satisfaction with the novel PO-SST delivery system used in the IBSREST and in the IBSACT real-world study of patients with IBS. In addition, real-world usage data from the IBSACT revealed that 60.8% of the patients using open label PO-SST only needed 1 to 2 capsules per day to achieve adequate relief from IBS symptoms, which is considerably less than the 6 daily capsules patients received in IBSREST. In the IBSACT, overall satisfaction with PO-SST was 85% or higher, even with lower usage of PO-SST capsules compared to the per protocol dose used in IBSREST. In addition, the responses for all questions from patients in each study were remarkably similar despite the lower capsule intake by patients in the IBSACT population (Table 1).
Patients with severe IBS are historically not satisfied with treatment even in the presence of improvement of individual IBS symptoms [5]. Patients recruited for the IBSREST exhibited moderate to severe IBS symptoms defined by average daily IBSrelated abdominal pain scores of 4 or higher on a scale of 0 to 10 and Total IBS Symptom scores of 2 or higher on a scale of 0 to 4 [13]. Thus, an overall satisfaction rate of greater than 80% for POSST for all survey questions is promising.
The current analysis is subject to some other limitations; including the subjective nature of the data collection and reliance on patient recall for both the IBSREST end-ofstudy questionnaire and the IBSACT survey. The surveys relied on the patients’ perception of improvement rather than physician measurement of improvement. In addition, although the IBSACT survey collected data on the frequency of IBS symptoms as reported by the patient, the severity of symptoms was not assessed and the demographics of patients was not collected. Adverse event data was not collected in the IBSACT survey. However, PO-SST was well tolerated and the adverse event rate was low in the IBSREST study in patients that received 6 capsules of PO-SST daily [13]. Finally, since the design of the IBSACT was as a post-marketing study, there is the possibility that patients who did not experience a clinical improvement with PO-SST may have been less likely to respond to the surveys included with the PO-SST provided to them, possibly leading to inflated estimates of patient satisfaction. It is striking, however, that patients with IBS in a RCT as well as in an open-label post-marketing IBS population reported a similar degree and onset of symptomatic relief and overall improvement in IBS symptoms and patientperceptions in changes in their quality of life while taking POSST. The majority of patients taking PO-SST reported overall improvement, especially in abdominal pain, cramping, and bowel regularity, making PO-SST an option for patients who desire rapid relief of IBS symptoms.
Competing Interests
Brooks D. Cash, MD, is a consultant for IM HealthScience, LLC. Michael S. Epstein, MD, is the Chief Medical Advisor for IM HealthScience, LLC. Syed M. Shah, PhD. is the Chief Innovation Officer at IM HealthScience, LLC.
This study was funded by IM HealthScience, LLC.
Author Contributions
BC is the guarantor of the article and contributed to data acquisition, data analysis, drafting of manuscript, and critical revision of manuscript. I was involved in study design, implementation, data acquisition, data analysis, drafting of manuscript, and critical revision of manuscript. SS contributed to study design, implementation, data acquisition, data analysis, drafting of manuscript, and critical revision of manuscript.
Acknowledgements, Funding, and Disclosures
The authors thank the principal investigators on the trials: Dennis S Riff, MD, FACG, CPI; Steven C Bowman, MD; Gigi Claire Lefebvre, MD; and Richard Krause, MD; Palm Beach CRO, LLC for help conducting the trial; SDC Biostatistics and Data Management for providing power and statistical analyses; Hubbell Consulting, LLC for preparing the clinical study report; and Whitney Smalley- Freed, PhD, for editorial support. Medical writing support was provided my Precise Medical Writing, LLC.
References
- Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L (2007) Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo-controlled randomized trial. Dig Liver Dis 39:530-536.
- Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndromeEuropean Medicines Agency (2014).
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, et al. (2006) Functional bowel disorders. Gastroenterology 130:1480-1491.
- Canavan C, West J, Card T (2014) Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther40:1023-1034.
- Whitehead WE, Palsson OS, Levy RL, Feld AD, VonKorff M, et al. (2006) Reports of "satisfactory relief" by IBS patients receiving usual medical care are confounded by baseline symptom severity and do not accurately reflect symptom improvement. Am J Gastroenterol101:1057-1065.
- Hawthorn M, Ferrante J, Luchowski E, Rutledge A, Wei XY, et al. (1988) The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment Pharmacol Ther 2:101-118.
- Walstab J, Wohlfarth C, Hovius R, Schmitteckert S, Roth R, et al. (2014) Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: implications for treating gastrointestinal disorders. NeurogastroenterolMotil 26:810-820.
- Juergens UR, Stober M, Vetter H (1998)The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: a novel perspective for its therapeutic use in inflammatory diseases. Eur J Med Res 3:539-545.
- Hawrelak JA, Cattley T, Myers SP (2009) Essential oils in the treatment of intestinal dysbiosis: A preliminary in vitro study. Altern Med Rev 14:380-384.
- Galeotti N, Di Cesare ML, Mazzanti G, Bartolini A, Ghelardini C (2002) Menthol: a natural analgesic compound. Neurosci Lett 322:145-148.
- Harries N, James KC, Pugh WK (1977) Antifoaming and carminative actions of volatile oils. Journal of Clinical Pharmacy and Therapeutics 2:171-177.
- Grigoleit HG, Grigoleit P (2005)Gastrointestinal clinical pharmacology of peppermint oil. Phytomedicine 12:607-611.
- Cash BD, Epstein MS, Shah SM (2015) A Novel Delivery System of Peppermint Oil Is an Effective Therapy for Irritable Bowel Syndrome Symptoms DigDisSci.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences