Celiac Disease in Children: A Review
Cecilia Mantegazza,GianVincenzo Zuccotti,Dario Dilillo,Jutta Koglmeier
DOI10.4172/2472-1891.100009
Cecilia Mantegazza1*, GianVincenzo Zuccotti1, Dario Dilillo1 and Jutta Koglmeier2
1Department of Pediatrics, University of Milan, Ospedale dei Bambini Vittore Buzzi, Milan, Italy
2Great Ormond Street Hospital for Children NHS Foundation Trust London, United Kingdom
- *Corresponding Author:
- Cecilia Mantegazza
Department of Pediatrics, University of Milan
Ospedale dei Bambini Vittore Buzzi, Milan, Italy
Tel: 02-57991
E-mail: cecimante@hotmail.com
Received date: September 25, 2015, Accepted date: October 31, 2015, Published date: November 07, 2015
Citation: Mantegazza C, et al. Celiac Disease in Children: A Review. Int J Dig Dis. 2015, 1:1. doi:10.4172/2472-1891.100009
Abstract
Celiac disease is a lifelong immune-mediated systemic disorder that may develop in genetically predisposed individuals when exposed to dietary gluten. Its prevalence is estimated to be 1-3% in the European Community; in particular in children studies report a prevalence ranging from 1/500 to 1/93. Awareness of its wide clinical spectrum is mandatory and relevant to all physicians, and in particular pediatricians, to allow a prompt diagnosis and therapy. While the first european guidelines recommended the need of 3 consecutive duodenal biopsies to establish a diagnosis, from 2012 an histological assessment is not considered necessary in certain circumstances in children. Gluten free diet is still the only therapy available in celiac disease disease but dietary adherence allows control of symptoms and reduces the risk of malignancy. However this diet is challenging due to its costs, quality of life implications, and health consequences such as obesity. Therefore alternative therapies are currently being developed. This review will focus on the current knowledge on celiac disease in children.
Summary
Celiac disease is a lifelong immune-mediated systemic disorder that may develop in genetically predisposed individuals when exposed to dietary gluten. Its prevalence is estimated to be 1-3% in the European Community; in particular in children studies report a prevalence ranging from 1/500 to 1/93. Awareness of its wide clinical spectrum is mandatory and relevant to all physicians, and in particular pediatricians, to allow a prompt diagnosis and therapy. While the first european guidelines recommended the need of 3 consecutive duodenal biopsies to establish a diagnosis, from 2012 an histological assessment is not considered necessary in certain circumstances in children. Gluten free diet is still the only therapy available in celiac disease disease but dietary adherence allows control of symptoms and reduces the risk of malignancy. However this diet is challenging due to its costs, quality of life implications, and health consequences such as obesity. Therefore alternative therapies are currently being developed. This review will focus on the current knowledge on celiac disease in children.
Abbreviations
CD: Celiac Disease; GFD: Gluten Free Diet
Definition and Prevalence
Celiac disease (CD) was first described in 1887 and is a lifelong immune-mediated multi-system disease. It may develop in genetically susceptible individuals when exposed to gluten and related prolamins. The diagnosis is based on the presence of gluten induced clinical symptoms, CD-specific antibodies and enteropathy [1-4]. Recent studies have shown that the prevalence of CD is much higher than previously recognised. In Europe 1-3% of the total population is affected [1]. Several paediatric studies have shown similar results in children with a ranging prevalence from 1/500 to 1/93 [5-10] and a higher incidence in the female gender [11]. In the past few decades the average age at diagnosis has risen from <2 years up to 6-9 years in many developed countries [11].
However, CD is still underdiagnosed; for every child with confirmed CD, there are seven other children with unrecognised and therefore untreated disease [1,5,12]. Clinicians are hence advised to have a low threshold for investigating symptomatic children or those with associated conditions [13].
Pathogenesis
A complex interplay between genetic, environmental and immunological factors plays a crucial role in the pathogenesis of CD [14].
In the 1940s Dicke identified gluten as the environmental trigger of CD. Gluten is a heterogeneous protein whose toxic fractions are a mixture of alcohol-soluble proteins called gliadins, which are found in cereals such as wheat, barley, rye consumed in most countries [15]. Gut enzymes are not capable of digesting gliadin fractions. Large proline/glutamine peptides therefore accumulate in the small intestinal lumen and may lead to a pathological innate and adaptive immune responses in genetically predisposed subjects [16].
Multiple genes are held responsible for the pathogenesis of CD: 97% of affected individuals carry at least one of the two human leukocyte antigen (HLA) class II genes DQ2 or DQ8 on chromosome 6p21; more than 90% have the DQ2 gene. These are responsible for the production of surface molecules expressed on the gut antigen presenting cells which bind gliadin peptides which in turn leads to a gliadin specific immune response through activation of CD4+ T cells [17]. The HLA-DQ2 and DQ8 genes are necessary but not sufficient for the development of CD [18,19]; thirty-nine non-HLA loci have been also found to contribute to the development of the disease, although to a much lesser degree (5% versus 35 %) [18,19]. The activation of CD4+T cells stimulate the release of proinflammatory cytokines and the production of specific antibodies such as anti-tissue transglutaminase (anti-tTG ), endomysial antibodies (EMA) and antibodies agains deaminated forms of gliadin peptides (DGP) [20]. In addition to gluten ingestion, multiple environmental factors have been linked to the development of CD in genetically predisposed children such as infections in early childhood, the gut microbiota in infants, feeding pattern and amount and timing of the initial introduction of gluten into the diet [21]. In particular associations between intestinal dysbiosis and CD has been demonstrated with microbiota imbalances observed not only in untreated CD patients but also in patients on a GFD. Microbiota alterations could play both a primary role by contributing to disease onset and a secondary role by aggravating CD pathogenesis [22].
Clinical Presentation
Since its first description in 1887 the understanding of CD is greatly improved. Atypical symptoms may be much more common than the classical ones. CD is not just a condition limited to the gastrointestinal tract and awareness of its huge clinical spectrum amongst clinicians is essential for a timely diagnosis and initiation of a gluten free diet [23]. In the medical literature ‘typical’ and ‘atypical’ CD have become obsolete and are replaced by classical and non classical CD [24]. Silent CD occurs in asymptomatic patients who have CD specific antibodies and duodenal biopsy findings in keeping with CD. Latent CD is seen in individuals with a CD compatible HLA status with a past history of glutendependent enteropathy but no current histological changes who may or may not be symptomatic and may be positive or negative for CD specific antibodies. Potential CD refers to patients with CD specific antibodies, compatible HLA type but normal small bowel biopsy in the presence or absence of symptoms. A gluten dependant enteropathy may develop at later stage [1].
In children classical CD is commonly diagnosed in the first 2 years of life. It is characterized by signs and symptoms of malabsorption such as chronic diarrhea, steatorrhea, failure to thrive and weight loss, stunted growth, muscle wasting, poor appetite, nausea and vomiting leading to lethargy and emotional distress. [23,25,26]. Other milder abdminal symptoms are currently more often described in CD such as abdominal pain, abdominal distention, flatulence, irregular bowel habits and chronic constipation [1]. In particular the last one is normally not responsive to standard therapy and is described in up to 25-30% of children [27-29].
Celiac crisis, once common, is a potentially life threatening complication of CD that is now rarely seen. It presents with profuse diarrhea leading to eletrolyte imbalances, severe dehydration and shock, abdominal distention, pedal edema, carpopedal spasm due to hypocalcaemia, muscle and buttock wasting, head drop, inability to stand and bleeding diathesis. If left untreated celiac crisis can lead to shock and eventually death [30].
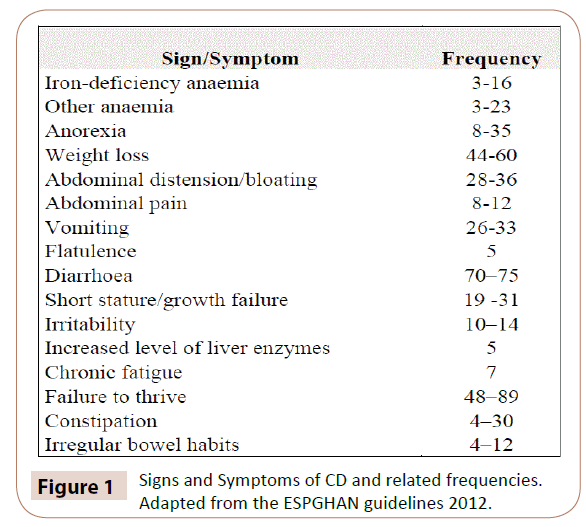
Non classical CD is mostly diagnosed in older children and adolescents presenting with extra-intestinal symptoms associated with the disease, such as puberty delay, unexplained chronic or iron deficient anaemia non responsive to supplementation, decreased bone mineralisation (osteopenia/osteoporosis), dental enamel defects, irritability, chronic fatigue, neuropathy, arthritis/arthralgia, amenorrhea, unexplained increased liver enzymes and recurrent aphthous stomatitis [1,25,26]. Moreover CD can present with a well-recognized skin manifestation, named dermatitis herpetiformis, which is characterized by pruritic papular eruption over the extensor surfaces around the elbows, knees, and buttocks, with a characteristic subepithelial deposition of IgA at the histological examination [1,25,31]. Signs and symptoms of CD and related frequencies are reported in (Figure 1).
The recent advances in serological testing have allowed many patients to be diagnosed when still asymptomatic but at risk of developing CD due to a CD associated condition or first degree relative in the family. According to reports only 1:3 to 1:7 of CD sufferers are symptomatic. [32]. A number of conditions have been associated with CD including type I diabetes (life-time prevalence ≥ 8%), selective IgA deficiency (1.7%–7.7%), Trisomy 21 (5%–12%), Williams (8.2%) and Turner syndrome (4.1%–8.1%), autoimmune thyroiditis (∼ 15%) and autoimmune liver disease (12-13%) [1]. First degree relative of an index case have 10% chance of developing CD at some point in their life, HLA matched siblings 30 to 40% and monozygotic twin 70 % higlighting the strong genetic link [13]. CD should also be ruled out in children with juvenile idiopathic arthritis, epilepsy characterized by intracranial calcification and in those with unexplained neurological problems such as palsies, neuropathies, or migraine [13].
Diagnosis
In 1969 the the European Society of Pediatric Gastroenterology and Nutrition (ESPGHAN) established the first criteria for the diagnosis of CD: Three consecutive biopsies with relapse of the histological changes when the patient was challenged with gluten after a strict gluten free diet were deemed necessary to confirm the diagnosis [32]. Since then the diagnostic criteria have been modified several times, particularly since serological testing has become widely available.
In children the correlation between significantly elevated tTG IgA levels and CD compatible duodenal biopsy results [33-35] and the strong dependence of CD on the HLA DQ2-DQ8 haplotypes, has questionned the diagnostic necessity to perform an upper gastrointestinal endoscopy [36,37]. In 2012 the ESPGHAN working group on Celiac Disease Diagnosis published new guidelines on which current clinical practice in Europe is based [1]. The diagnostic approach differs quite significantly from the past.
Initial screening of children with both classical or non classical symptoms requires measurement of total IgA and anti-tTG IgA antibodies [1]. Negative anti-tTG IgA antibodies in the presence of a normal total IgA make a diagnosis of CD unlikely; however false negative anti-tTG IgA must be considered if the child’s diet is low in gluten, in the presence of a protein losing enteropathy, concommitant immunosuppressive therapy and in children younger than 2 years of age [1].
When anti-tTG IgA antibodies are ten times or more above the normal range, EMA IgA should be checked and HLADQ2/DQ8 typing performed. A diagnosis of CD without need for duodenal biopsy can be made if both results are positive. Upper GI endoscopy is recommended if one result is not in keeping with CD [1]. A false positive tTG IgA should be considered if both results are negative [1].
Duodenal biopsies are required if anti-tTG IgA antibodies are less then ten times above the normal range [1]. CD may be patchy and four biopsies from the second part of the duodenum or more distally and one or two biopsies from the dudodenal bulb are hence recommended [1]. An adequate gluten intake at the time of duodenal biopsy is essential for reliable results [1].
The histological findings according to the Marsh-Oberhuber classification is based on the degree of villous atrophy, crypt elongation, villus-crypt ratio and the number of intraepithelial lymphocytes. A Marsh-Oberhuber grading of 2–3 is consistent with CD [1].
More challenging clinical situations in symptomatic children are also addressed in the guidelines.
• In the presence of IgA deficiency, at least one additional test using IgG class specific antibodies should be performed (anti tTG or anti EMA or antibodies against DGP) which may help to decide if to proceed to duodenal biopsy or not. However, as IgG antibodies have a lower specificity, a biopsy may be still indicated if the IgG antibodies come back negative [1].
• CD in children with dermatitis herpetiformis can be confirmed by immunofluorescent examination of the skin showing IgA deposits in the dermis [1]. Approximately 80% of patients with this skin alteration show villous atrophy in the small bowel mucosa at the time of the diagnosis.
• Children younger than 2 years of age should be tested both for IgA and IgG CD specific antibodies after a trial of a cow’s milk protein free diet. DGP antibodies may be helpful as they have a potentially higher sensitivity than EMA and anti-tTG although they are less specific. A duodenal biopsy should be performed when there is a strong clinical suspicion of CD [1].
In asymptomatic children with an increased risk to develop CD, HLA typing can be useful. The development of CD is unlikely in the absence of a HLA DQ2 or DQ8 positive genotype and serological screening is only justified in symptomatic children. Surveillance serological screening every three years can be performed in asymptomatic children who are HLA DQ2/DQ8 positive [1].
Gluten Free Diet
A strict gluten-free diet (GFD) for life is currently the only available treatment for CD [38].
Children should be started on a GFD only once the diagnosis has been made in order to avoid unnecessary dietary restrictions and diagnostic uncertainty; however in very symptomatic patients presenting in poor clinical condition or in celiac crisis, when treatment cannot safely be delayed, GFD can be considered prior to a full diagnostic work-up [1].
Gluten free labelled foods, beverages and medication have a gluten content of less than 20 part per million. A gluten intake of less than 10 mg per day is considered safe for patients with CD [39]. All gluten containing grains such as wheat, rye, barley, spelled and kamut have to be avoided. Pure oats are tolerated by the majority of patients [40]
GFD in most cases leads to a rapid clinical improvement depending on symptoms, however it will take months or years to reach complete mucosal healing [41].
Poor dietary compliance may be complicated by osteoporosis, infertility or certain types of cancer such as small bowel adenocarcinoma, enteropathy associated T cell lymphoma [38], other types of non-Hodgkin’s lymphoma and Hodgkin’s lymphoma, recent studies have shown that cancer in patients with CD is than previously thought [42-44]. Patients who follow a strict GFD have no increased risk of comorbidity or malignancies. [45].
A GFD is often expensive and can negatively impact on quality of life (Makharia), making strict adherence difficult. Compliance is particularly poor amongst adolescents with rates as low as 52% and 81% at best making this a particularly vulnerable group of patients [46]. Adolescent Celiac clinics tailored to the needs of older children and young adults can help to address this problem.
The Canadian Celiac Association Health Survey conducted in 2003 on 168 children with biopsy-confirmed CD hihlighted many problems children face when starting a GFD: 92% reported difficulty in determining whether foods were gluten free, 90% struggled to find foods which were allowed, 95% complained about having to avoid eating out in restaurants, 46% of children did not travel away from home or abroad and 72% were angry about having to follow a special diet [47]. In addition the avoidance of gluten-containing staple foods can potentially lead to a loss of an important source of energy, proteins, carbohydrates and micronutrients with long term implications on health, nutritional status and growth [48]. Wheat-based refined gluten-free cereal products do not contain the same levels of thiamine, riboflavin, niacin, folate and iron compared to the equivalent gluten containing products and may further impact on a child’s nutritional intake [49]. Some studies have demonstrated that a GFD can be unbalanced, rich in fat and protein and poor in carbohydrate, which may contribute to obesity. Up to 15- 20% of patients with CD move from a normal or low BMI centile at diagnosis to a BMI considered overweight and up to 20% of those already overweight at diagnosis gain more weight on the diet [50,51]. In 2007 Zuccotti et al demonstrated that children on a GFD had significantly higher energy intakes than controls and consumed excessive intakes of simple sugars, fats and protein; however, the body mass index was similar in both groups. [52] This study also highlighted the lack of information on gluten free products’ nutritional contents, and therefore the difficulties encountered in a proper estimation of true fat and micronutrient intake of a child on a GFD [52]. Other studies suggest that patients with CD have a good nutritional status and normal BMI despite a diet often low in fiber and iron and high in satured fats, now common in the countries with high socio economic status [53].
Easy access to a dietitian experienced in the management of CD is crucial and children should receive age appropriate information about the implications of CD. The psychological impact of a CFD should not be underestimated and families supported as much as possible.
Novel Therapies
As children and adults with CD continue to report difficulties related to a life long GFD [54] novel therapeutic strategies with the potential to treat or even cure CD are being explored [14]. Current novel concepts involve dietary modification, permeability inhibition and mucosal reconstruction, antigen presentation suppression, cytokine therapy and anti inflammation, adhesion blockade and immunomodulation [55].
Although still in the preclinical stage new wheat variants are being developed either by reproduction of wheat species lacking harmful gluten epitopes or by genetical modification of the immunogenic peptides. It may be hence possible to avoid gluten in the human diet completely, an approach that may not only treat but also prevent CD ; however, commercially available wheat is a very cheap and robust industrial commodity, therefore it is unlikely that modified grains would replace commercial wheat strains [56,57]. Inhibition of mucosal permeability, an early pathogenic event in the development of CD which allows the passage of immunodominant gluten peptides and other immunostimulatory luminal antigens, has been used in trails. An octapeptide antagonizing Zonulin, a human protein which enhances epithelial permeability, has been used with good results [58]. In vitro studies using reversible and irreversible inhibitors of tissue transglutaminase 2 enzyme to block the deamidation process of gluten had mixed results and raised concerns about potential interaction with vital biologic pathways [59,60]. Analogous gliadin peptide, with enhanced affinity for DQ2 and able to inhibit HLADQ2 mediated antigen presentation, have been developed and may be a potential treatment for CD; however celiac-specific HLA inhibition must not interfere with class 2 dependent responses and immunosurveillance. [61,62] Different strategies targeting the cytokines and chemokines involved in the pathogenesis of CD as well as the associated chemokine receptors (anti IL-10 and IL-15, anti-Interferon-Gamma and TNF Alpha) are beeing explored. However while this approach may be justified in other auto-immune diseases such as inflammatory bowel disease, the potential side effects may not justify its use in CD where dietary elimination of gluten offers complete resolution of symptoms [55]. Tolerance induction has been demonstrated in mouse models, but lacks human studies. Probiotics have been trialled in CD due to their role in maintaining gut barrier function and regulating the response of the innate and adaptive immune system [22]. To date three randomized, double-blind placebocontrolled human intervention trials have been conducted in CD patients: B. infantis NLS was administered to untreated patients with an improvement in some gastrointestinal symptoms, in particular indigestion and constipation, but with no modification in intestinal permeability or in the pro-inflammatory status [63]; B. longum CECT 7347 was trialled in CD children on a GFD leading to a decrease in peripheral CD3+ T lymphocytes and a trend in the reduction of TNF serum levels [64]; B. breve BR03 and B. breve B632 were administered to children on a GFD with a reduction in pro-inflammatory cytokine TNF [65].
Finally a gluten vaccine has also been developed and completed a phase I clinical trial on HLA DQ2 positive volunteers with CD; the vaccine led to well tolerated immunization without any serious adverse events [55].
Although still far from being available in routine clinical practice, non diet-based therapies hold promise and may be available to patients who cannot or choose not to follow a GFD.
Follow-Up
Little is known from the literature how often and in what format children with CD should be monitored despite a consensus that CD is a chronic and potentially serious medical condition which requires long term follow up to guarantee adherance to therapy and prevent complications.
A Paediatrician familiar in the diagnosis and management of CD or a paediatric Gastroenterologist should review a child with CD every 6 months [45]. Older teenagers and young adults form a particularly challenging cohort of patients who may demonstrate poor adherance to therapy and miss clinic appointments and therefore require special attention.
Anti tTG IgA is checked to monitor compliance although data on the reliability of anti tTG IgA in the context is controversial [45]. Levels of anti tTG IgA should decline consistently over time and can take up to 1-1.5 years before being completely back to normal. Mucosal healing occurs generally in 3 to 6 months [45]. Routine surveillance upper endoscopies with intestinal biopsies are not recommended except in those cases with lack of clinical response or relapse of symptoms despite a correct GFD [66,67].
In the presence of ongoing weight loss other parameteres such as haemoglobine, total protein count, albumin, iron, vitamin B6, folic acid, vitamin B12, calcium, alkaline phosphatase, vitamin D, parathyroid hormone may be helpful. Routine screening for other autoimmune disorders (Thyroid-stimulating hormone, Thyroid hormone, liver function tests, glucose) is justifiable and can be done from time to time [45].
Anthroprometric parameters should be checked at every visit and BMI recorded. Bone density measurement is not routinely suggested in children but can be considered [45].
At diagnosis screening (DQ2/D8 and celiac serology) should be offered to first degree relatives and membership in a celiac support group encouraged.
Conclusion
CD is estimated to affect 1-3% of children in the European community; while typical symptomatic patients are nowadays a minority, several mild or non gastrointestinal symptoms are currently more common and moreover asymptomatic children form a majority of the new diagnosed cases. Awareness on CD’s huge clinical spectrum is hence mandatory and a low threshold for investigating symptomatic children or those with associated conditions is recommended. Duodenal biopsy used to be the gold standard to confirm the diagnosis but can now be avoided in a number of cases highlighted in the 2012 guidelines. GFD remains the only therapeutic option to induce remission but novel therapies may offer alternatives in the future for those who struggle with a GFD.
References
- Husby S, Koletzko S, Korponay-Szábó IR, Mearin ML, Phillips A, et al. (2012) ESPGHAN guidelines for the diagnosis of coeliac disease in children and adolescents. An evidence-based approach 54:136–160.
- Abadie V, Sollid LM, Barreiro LB, Jabi B (2011) Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol 29:493–525.
- Green PHR, Jabri B (2003) Coeliac Disease. Lancet 362:383–391.
- Kaukinen K, Partanen J, Maki M, Collin P (2002) HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol 97:695–699.
- Schuppan D (2000) Gastroenterology 119: 234–242.
- Csizmadia CGDS, Mearin ML, von Blomberg BME, Brand R, Verloove-Vanhorick SP (1999) An iceberg of childhood coeliac disease in the Netherlands. Lancet 353:813-814.
- Carlsson AK, Axelsson IE, Borulf SK, Bredberg AC, Ivarsson SA (2001) Serological screening for celiac disease in healthy 2.5-year-old children in Sweden. Pediatrics 107:42-45.
- Maki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, et al. (2003) Prevalence of Celiac disease among children in Finland. N Engl J Med 348: 2517-2524.
- Tommasini A, Not T, Kiren V, Baldas V, Santon D, et al. (2004) Mass screening for coeliac disease using antihuman trans- glutaminase antibody assay. Arch Dis Child 89:512-515.
- Catassi C, Ra¨tsch IM, Fabiani E, Rossini M, Bordicchia F, et al. (1994) Coeliac disease in the year 2000: exploring the iceberg. Lancet 343:200-203.
- Castan o L, Blarduni E, Ortiz L, Nu´n ez J, Bilbao JR, et al. (2004) Prospective population screening for celiac disease: high prevalence in the first 3 years of life. J Pediatr Gastroenterol Nutr 39:80-84.
- Kivela L, Kaukinen K, Lahdeaho M, Huhtala, H Ashorn M, et al. (2015) Presentation of Celiac Disease in Finnish Children Is No Longer Changing:A 50-Year PerspectiveJ Pediatr.
- Fasano A, Catassi C (2001) Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology 120: 636-651.
- Murch S, Jenkins H, Auth M, Bremner R, Butt A, et al. (2013) Joint BSPGHAN and Coeliac UK guidelines for the diagnosis and management of coeliac disease in children. Arch Dis Child 98:806-811.
- Schuppan D, Junker Y, Barisani D (2009) Celiac disease: from pathogenesis to novel therapies. Gastroenterology 137: 1912-1933.
- Van B H GP, Mulder CJ (1993) Pioneer in the gluten free diet: Willem-Karel Dicke1905-1962, over 50 years of gluten free diet. Gut 34:1473-1475.
- Hausch F, Shan L, Santiago NA, Gray GM, Khosla C (2002) Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 283:G996-G1003.
- Molberg O, Kett K, Scott H, Thorsby E, Sollid LM, et al.(1997) Gliadin specific, HLA DQ2-restrictedT cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand J Immunol 46:103-109.
- Dubois P C A, Trynka G,Franke L, Hunt KA, Romanos J, et al. (2010) Multiple common variants for celiac disease influencingimmune gene expression. Nature Genetics 42: 295–302.
- Trynka G, Wijmenga C, van Heel D A (2010) A genetic perspective on coeliac disease,Trends in Molecular Medicine 16: 537–550.
- Abadie V, Sollid LM, Barreiro LB, Jabri B (2011) Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol 29:493-525.
- Guandalini S, Assiri A (2014)Celiac disease: a review. JAMA Pediatr 168: 272-278.
- Cenit MC, Olivares M, Codoner F P, Sanz Y (2015) Intestinal Microbiota and Celiac Disease: Cause, Consequence or Co-Evolution? Nutrients 7: 6900-6923.
- Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P et al. (2007) Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther 26:1217–1225.
- Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A,et al. (2013) The Oslo definitions for coeliac disease and related terms. Gut 62:43–52.
- Di Sabatino A, Corazza GR (2009)Coeliac disease. Lancet 373:1480-1493.
- McGowan KE, Castiglione DA, Butzner JD (2009) The changing face of childhood celiac disease in north america: impact of serological testing. Pediatrics 124:1572-1578.
- Khatib M, Baker RD, Ly EK, Kozielski R, Baker SS (2015) The Presenting Pattern of Pediatric Celiac Disease. J Pediatr Gastroenterol Nutr.
- Wagner G, Berger G, Sinnreich U, Grylli V, Schober E, et al. (2008) Quality of life in adolescents with treated coeliac disease: influence of compliance and age at diagnosis. J Pediatr Gastroenterol Nutr 47: 555-561.
- Kokkonen J,Viitanen A,Simila S (1989) Copingwithacoeliac diet after adolescence. Helv Paediatr Acta 43:261-265.
- Baranwal AK, Singhi SC, Thapa BR, Kakkar N (2003)Celiac crisis. Indian J Pediatr 70:433-435.
- Antiga E, Caproni M (2015) The diagnosis and treatment of dermatitis herpetiformis. Clin Cosmet Investig Dermatol 8:257-265.
- Mustalahti K, Sulkanen S, Holopainen P, Laurila K, Collin P, et al. (2002) Coeliac disease among healthy members of multiple case coeliac disease families. Scand J Gastroenterol 37:161-165.
- Meeuwisse GW (1970) Diagnostic criteria in coeliac disease. Acta Paediatr Scand 59: 461-463.
- Barker CC, Mitton C, Jevon G, Mock T (2005) Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics 115:1341–1346.
- Donaldson MR, Firth SD, Wimpee H, Leiferman KM, Zone JJ, et al. (2007) Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol 5:567–573.
- Mearin ML, Ribes K C, Biemond I, Polanco I, Pena AS (1984) Influence of genetic factors on the serum levels of antigliadin antibodies in celiac disease. J Pediatr Gastroenterol Nutr 3: 373-377.
- Margaritte J P, Babron MC, Bourgey M, Louka AS, Clot F, et al. (2004) HLA-DQ relative risks for coeliac disease in European populations: a study of the European Genetics Cluster on Coeliac Disease. Tissue Antigens 63:562–567.
- Green PHR, Jabri B (2003) Coeliac disease. Lancet 362:383-391.
- Akobeng AK, Thomas AG (2008) Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther 27: 1044-1052.
- Pulido OM, Gillespie Z, Zarkadas M, Dubois S, Vavasour E et al. (2009) Introduction of oats in the diet of individuals with celiac disease: a systematic review. Adv Food Nutr Res 57:235.
- Wahab PJ, Meijer JW, Mulder CJ (2002) Histologic follow-up of people with celiac disease on a gluten-free diet: slow andincomplete recovery. Am J Clin Pathol 118:459.
- Catassi C, Fabiani E, Corrao G, Barbato M, De Renzo A, et al. (2002) Risk of non-Hodgkin lymphoma in celiac disease. JAMA 287: 1413.
- Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, et al. (2002) Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 123: 1428-1435.
- Mearin ML, Catassi C, Brousse N, Brand R, Collin P,et al. (2006) European multi-centre study on coeliac disease and non-Hodgkin lymphoma. Eur JGastroenterol Hepatol 18: 187-194.
- Mulder C J, Wierdsma N J, Berkenpas M, Jacobs M A J M, Bouma G (2015) Preventing complications in celiac disease: Our experience with managing adult celiac disease. Best Practice & Research Clinical Gastroenterology 29: 459-468.
- Fabiani E, Catassi C, Villari A, Gismondi P, Pierdomenico R, et al. (1996) Dietary compliance inscreening-detected coeliac disease adolescents. Acta Paediatr 412: 65- 67.
- Rashid M, Cranney A, Graham I, Zarkadas M, Switzer C, et al. (2003) Canadian celiac health survey: pediatric data. J Pediatr Gastroenterol Nutr 37:A127.
- Whitton C, Nicholson S K, Roberts C, Prynne C J, Pot GK, et al. (2011) National Diet and Nutrition Survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br. J. Nutr 7: 1-16.
- Thompson T (1999) Thiamine, riboflavin, and niacin contents of gluten-free diet. J. Am. Diet. Assoc 99: 858–862.
- Kabbani TA, Goldberg A, Kelly CP, Pallav K, Tariq S, et al. (2012) Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment Pharmacol Ther 35: 723-729.
- Mariani P, Viti MG, Montuori M, La Vecchia A, Cipolletta E, et al. (1998) The gluten-free diet: a nutritional risk for adolescents with celiac disease. J Pediatr Gastroenterol Nutr 27: 519-523.
- Zuccotti G, Fabiano V, Dilillo D, Picca M, Cravidi C,et al. (2013) Intakes of nutrients in Italian children with celiac disease and the role of commercially available gluten-free products. J Hum Nutr Diet 26: 436–444.
- Hopman EG, le Cessie S, von Blomberg BM, Mearin ML (2006) Nutritional management of the gluten-free diet in young people with celiac disease in The Netherlands. J Pediatr Gastroenterol Nutr. 43:102-108.
- Aziz I, Evans KE, Papageorgiou V, Sanders DS (2011) Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? J Gastrointestin Liver Dis 20:27-31.
- Rashtak S, Murray J. A (2012) Review Article: Celiac Disease, New Approaches to Therapy. Aliment Pharmacol Ther 35: 768-781.
- Zandonadi RP, Botelho RB, Araujo WM (2009) Psyllium as a substitute for gluten in bread. J Am Diet Assoc 109:1781–1784.
- Stenman SM, Lindfors K, Venalainen JI, Hautala A, Mannisto PT, et al. (2010) Degradation of coeliac disease-inducing rye secalin by germinating cereal enzymes: diminishing toxic effects in intestinal epithelial cells. Clin Exp Immunol 161: 242-249.
- Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB (2007) The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 26:757–766.
- Klock C, Jin X, Choi K, Khosla C, Madrid PB,et al. (2010) Acylideneoxoindoles: A new class of reversible inhibitors of human transglutaminase 2. Bioorg Med Chem Lett.
- Van de Wal Y, Kooy YM, van Veelen P, Vader W, August SA, et al. (1999) Glutenin is involved in the gluten-driven mucosal T cell response. Eur J Immunol 10:3133–3139.
- Xia J, Bergseng E, Fleckenstein B, Siegel M, Kim CY et al. (2007) Cyclic and dimeric gluten peptide analogues inhibiting DQ2-mediated antigen presentation in celiac disease. Bioorg Med Chem 15: 6565-6573.
- Xia J, Siegel M, Bergseng E, Sollid LM, Khosla C (2006) Inhibition of HLA-DQ2-mediated antigen presentation by analogues of a high affinity 33-residue peptide from alpha2-gliadin. J Am Chem Soc. 128:1859–1867.
- Smecuol E, Hwang H J, Sugai E, CorsoL, Chernavsky A C, et al. (2013) Exploratory, randomized, double-blind, placebo-controlled study on the effects of bifidobacterium infantis natren life start strain super strain in active celiac disease. J. Clin. Gastroenterol 47: 139-147.
- Olivares M, Castillejo G, Varea V, Sanz Y (2014) Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of bifidobacterium longum cect 7347 in children with newly diagnosed coeliac disease. Br. J. Nutr 112: 30-40.
- Klemenak M, Dolinsek J, Langerholc T, Di Gioia D (2015) Administration of Bifidobacterium breve Decreases the Production of TNF- in Children with Celiac Disease. Dig. Dis. Sci. 2015.
- RubioTA, Hill ID, Kelly CP, Calderwood AH, Murray JA (2013) American college of gastroenterology clinical guideline: diagnosis and management of celiac disease. Am J Gastroenterol 108: 656-677.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences